当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An organocatalytic asymmetric Mannich reaction for the synthesis of 3,3-disubstituted-3,4-dihydro-2-quinolones†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-07-11 00:00:00 , DOI: 10.1039/c8ob01399c Soumendranath Mukhopadhyay 1, 2, 3 , Subhas Chandra Pan 1, 2, 3
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-07-11 00:00:00 , DOI: 10.1039/c8ob01399c Soumendranath Mukhopadhyay 1, 2, 3 , Subhas Chandra Pan 1, 2, 3
Affiliation
|
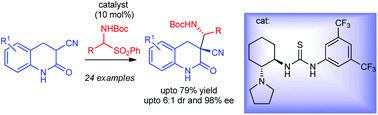
The first organocatalytic asymmetric Mannich reaction employing 3,4-dihydro-2-quinolones has been developed for the synthesis of biologically important 3,3-disubstituted-dihydro-2-quinolones. N-Boc imine precursor amidosulfones as well as pre-formed N-Boc imine were used for this purpose. Cyclohexyldiamine derived bifunctional amino-thiourea catalysts were employed to provide the products in high enantio- and good diastereoselectivities.
中文翻译:

有机催化不对称曼尼希反应,用于合成3,3-二取代-3,4-二氢-2-喹诺酮†
已经开发了使用3,4-二氢-2-喹诺酮的第一个有机催化不对称曼尼希反应,用于合成生物学上重要的3,3-二取代-二氢-2-喹诺酮。N- Boc亚胺前体酰胺砜以及预先形成的N - Boc亚胺均用于此目的。使用环己基二胺衍生的双官能氨基-硫脲催化剂可提供高对映异构性和良好的非对映选择性的产物。
更新日期:2018-07-11
中文翻译:

有机催化不对称曼尼希反应,用于合成3,3-二取代-3,4-二氢-2-喹诺酮†
已经开发了使用3,4-二氢-2-喹诺酮的第一个有机催化不对称曼尼希反应,用于合成生物学上重要的3,3-二取代-二氢-2-喹诺酮。N- Boc亚胺前体酰胺砜以及预先形成的N - Boc亚胺均用于此目的。使用环己基二胺衍生的双官能氨基-硫脲催化剂可提供高对映异构性和良好的非对映选择性的产物。









































 京公网安备 11010802027423号
京公网安备 11010802027423号