当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recent advances in the application of Diels–Alder reactions involving o-quinodimethanes, aza-o-quinone methides and o-quinone methides in natural product total synthesis
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2018-07-11 00:00:00 , DOI: 10.1039/c8cs00274f Baochao Yang 1, 2, 3, 4, 5 , Shuanhu Gao 1, 2, 3, 4, 5
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2018-07-11 00:00:00 , DOI: 10.1039/c8cs00274f Baochao Yang 1, 2, 3, 4, 5 , Shuanhu Gao 1, 2, 3, 4, 5
Affiliation
|
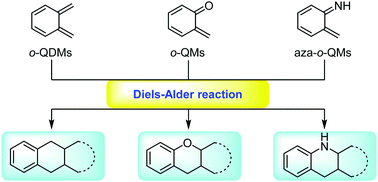
In recent decades, transient and highly reactive ortho-quinodimethanes (o-QDMs), ortho-quinone methides (o-QMs) and aza-ortho-quinone methides (aza-o-QMs) have attracted much attention and have been extensively studied and applied in organic synthesis, especially natural product total synthesis. This review summarizes recent advances in Diels–Alder reactions involving in situ-generated o-QDMs, o-QMs and aza-o-QMs, highlighting the power and potential of this strategy in organic synthesis and natural product total synthesis. An overview of the methods for generating these intermediates is also available.
中文翻译:

涉及邻喹二甲烷,氮杂邻醌醌和邻醌甲基醚的狄尔斯-阿尔德反应在天然产物全合成中 应用的最新进展
在最近的几十年中,瞬态和高反应性的邻苯二甲甲烷(o -QDMs),邻苯二甲酮甲基化物(o -QMs)和氮杂-邻苯醌甲基化物(aza- o -QMs)引起了广泛的关注,并得到了广泛的研究。用于有机合成,特别是天然产物的全合成。本文总结的Diels-Alder反应涉及的最新进展原位-生成Ø -QDMs,ø -QMs和氮杂Ø-QM,突出了该策略在有机合成和天然产物全合成中的作用和潜力。还提供了有关生成这些中间体的方法的概述。
更新日期:2018-07-11
中文翻译:

涉及邻喹二甲烷,氮杂邻醌醌和邻醌甲基醚的狄尔斯-阿尔德反应在天然产物全合成中 应用的最新进展
在最近的几十年中,瞬态和高反应性的邻苯二甲甲烷(o -QDMs),邻苯二甲酮甲基化物(o -QMs)和氮杂-邻苯醌甲基化物(aza- o -QMs)引起了广泛的关注,并得到了广泛的研究。用于有机合成,特别是天然产物的全合成。本文总结的Diels-Alder反应涉及的最新进展原位-生成Ø -QDMs,ø -QMs和氮杂Ø-QM,突出了该策略在有机合成和天然产物全合成中的作用和潜力。还提供了有关生成这些中间体的方法的概述。











































 京公网安备 11010802027423号
京公网安备 11010802027423号