当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radical-mediated direct C–H amination of arenes with secondary amines†
Chemical Science ( IF 7.6 ) Pub Date : 2018-07-11 00:00:00 , DOI: 10.1039/c8sc01747f Sebastian C. Cosgrove 1, 2, 3, 4 , John M. C. Plane 1, 2, 3, 4 , Stephen P. Marsden 1, 2, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2018-07-11 00:00:00 , DOI: 10.1039/c8sc01747f Sebastian C. Cosgrove 1, 2, 3, 4 , John M. C. Plane 1, 2, 3, 4 , Stephen P. Marsden 1, 2, 3, 4
Affiliation
|
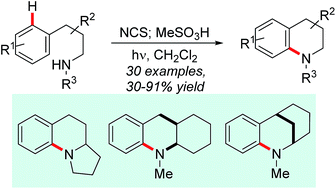
Aryl dialkyl amines, valuable subunits of a wide range of effect chemicals, are accessed by intramolecular amination of aromatic C–H bonds employing UV photolysis of N-chloroamines. The reactions show good functional group tolerance and allow access to a range of fused and bridged polycyclic structures. The homogeneous reaction conditions allow for the one-pot conversion of secondary amines to their arylated derivatives. Experimental and theoretical evidence supports the involvement of electrophilic aminium radicals which react via direct ortho-attack on the arene.
中文翻译:

自由基介导的芳烃与仲胺的直接C–H胺化†
芳基二烷基胺是广泛的作用化学物质的重要亚基,可通过使用N-氯胺的UV光解,通过芳族CH键的分子内胺化作用来获得。反应显示出良好的官能团耐受性,并允许进入一系列稠合和桥连的多环结构。均相反应条件允许将一元胺一锅转化为它们的芳基化衍生物。实验和理论证据支持亲电性铝自由基的参与,所述亲电性铝自由基通过直接邻位攻击在芳烃上反应。
更新日期:2018-07-11
中文翻译:

自由基介导的芳烃与仲胺的直接C–H胺化†
芳基二烷基胺是广泛的作用化学物质的重要亚基,可通过使用N-氯胺的UV光解,通过芳族CH键的分子内胺化作用来获得。反应显示出良好的官能团耐受性,并允许进入一系列稠合和桥连的多环结构。均相反应条件允许将一元胺一锅转化为它们的芳基化衍生物。实验和理论证据支持亲电性铝自由基的参与,所述亲电性铝自由基通过直接邻位攻击在芳烃上反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号