当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A highly site-selective radical sp3 C–H amination of azaheterocycles†
Chemical Science ( IF 7.6 ) Pub Date : 2018-07-10 00:00:00 , DOI: 10.1039/c8sc00590g Keith W. Bentley 1, 2, 3, 4 , Krysta A. Dummit 1, 2, 3, 4 , Jeffrey F. Van Humbeck 1, 5, 6, 7
Chemical Science ( IF 7.6 ) Pub Date : 2018-07-10 00:00:00 , DOI: 10.1039/c8sc00590g Keith W. Bentley 1, 2, 3, 4 , Krysta A. Dummit 1, 2, 3, 4 , Jeffrey F. Van Humbeck 1, 5, 6, 7
Affiliation
|
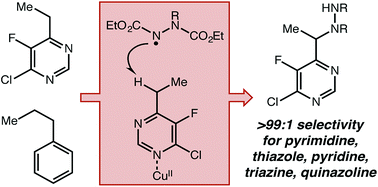
This report describes the development of a novel C–H amination strategy using both a Cu(II) Lewis acid and an organic hydrogen atom transfer catalyst to activate benzylic C–H bonds adjacent to aromatic N-heterocycles. This simple methodology demonstrates very high selectivity towards azaheterocycles without using exogenous directing groups and affords excellent site selectivity in substrates with more than one reactive position. A wide range of heterocyclic structures not compatible with previously reported catalytic systems have proven to be amenable to this approach. Mechanistic investigations strongly support a radical-mediated H-atom abstraction, which explains the observed contrast to known closed-shell Lewis acid catalyzed processes.
中文翻译:

氮杂杂环的 高度位点选择性自由基sp 3 C–H胺化†
该报告描述了一种新型的C–H胺化策略的开发,该策略同时使用Cu(II)路易斯酸和有机氢原子转移催化剂来激活与芳族N-杂环相邻的苄基C–H键。这种简单的方法显示了对氮杂杂环的非常高的选择性,而无需使用外源性的导向基团,并且在具有多个反应位置的底物中具有出色的位点选择性。与以前报道的催化体系不兼容的多种杂环结构已被证明适用于这种方法。机理研究强烈支持自由基介导的H原子抽象,这解释了与已知的闭壳Lewis酸催化过程所观察到的对比。
更新日期:2018-07-10
中文翻译:

氮杂杂环的 高度位点选择性自由基sp 3 C–H胺化†
该报告描述了一种新型的C–H胺化策略的开发,该策略同时使用Cu(II)路易斯酸和有机氢原子转移催化剂来激活与芳族N-杂环相邻的苄基C–H键。这种简单的方法显示了对氮杂杂环的非常高的选择性,而无需使用外源性的导向基团,并且在具有多个反应位置的底物中具有出色的位点选择性。与以前报道的催化体系不兼容的多种杂环结构已被证明适用于这种方法。机理研究强烈支持自由基介导的H原子抽象,这解释了与已知的闭壳Lewis酸催化过程所观察到的对比。











































 京公网安备 11010802027423号
京公网安备 11010802027423号