当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Reversible and Repeatable Thiol-Ene Bioconjugation for Dynamic Patterning of Signaling Proteins in Hydrogels.
ACS Central Science ( IF 12.7 ) Pub Date : 2018-07-12 , DOI: 10.1021/acscentsci.8b00325 Joseph C Grim 1, 2 , Tobin E Brown 1, 2 , Brian A Aguado 1, 2 , Douglas A Chapnick 3 , Alexandrea L Viert 1 , Xuedong Liu 3 , Kristi S Anseth 1, 2
ACS Central Science ( IF 12.7 ) Pub Date : 2018-07-12 , DOI: 10.1021/acscentsci.8b00325 Joseph C Grim 1, 2 , Tobin E Brown 1, 2 , Brian A Aguado 1, 2 , Douglas A Chapnick 3 , Alexandrea L Viert 1 , Xuedong Liu 3 , Kristi S Anseth 1, 2
Affiliation
|
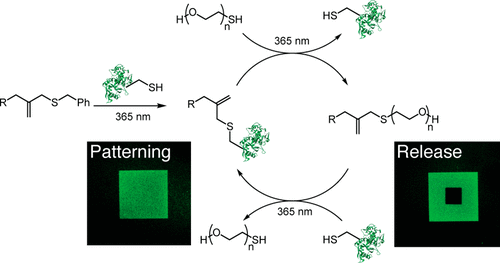
Biomolecule-functionalized hydrogels have emerged as valuable cell culture platforms to recapitulate the mechanical and biochemical properties of the extracellular niche. The typical strategy to functionalize hydrogels with biomolecules involves directly tethering them to the hydrogel backbone resulting in a static material. Thus, this approach fails to capture the dynamic changes in biomolecule composition that occur during biological processes or that may be required for regenerative medicine applications. Moreover, it also limits the scope of biomolecules to simple peptides, as signaling proteins generally have poor stability under cell culture conditions and lose their bioactivity over time. To that end, we sought to develop a bioconjugation reaction that would enable reversible and repeatable tethering of signaling proteins to hydrogels, so that spent protein could be released on-demand and replaced with fresh protein as needed. Specifically, we designed an allyl sulfide chain-transfer agent that enables a reversible, photomediated, thiol-ene bioconjugation of signaling proteins to hydrogels. Upon addition of a thiolated protein to the allyl sulfide moiety, the previously tethered protein is released, and the "ene" functionality is regenerated. Using this approach, we demonstrate that protein patterning can be achieved in hydrogels through a thiol-ene reaction, and the patterned protein can then be released through a subsequent thiol-ene reaction of a PEG thiol. Importantly, this process is repeatable through multiple iterations and proceeds at physiologically relevant signaling protein concentrations. Finally, we demonstrate that whole signaling proteins can be patterned and released in the presence of cells, and that cells respond to their presentation with spatial fidelity. Combined, these data represent the first example of a methodology that enables fully reversible and repeatable patterning and release of signaling proteins from hydrogels.
中文翻译:

可逆和可重复的硫醇-烯生物缀合物,用于水凝胶中信号蛋白的动态模式分析。
生物分子功能化的水凝胶已成为有价值的细胞培养平台,以概括细胞外生态位的机械和生化特性。用生物分子对水凝胶进行功能化的典型策略包括将它们直接束缚在水凝胶骨架上,从而产生静态物质。因此,该方法不能捕获生物分子组成在生物过程中发生的动态变化或再生医学应用可能需要的动态变化。此外,由于信号蛋白通常在细胞培养条件下稳定性差,并且随着时间的流逝失去其生物活性,这也将生物分子的范围限制为简单的肽。为此,我们寻求开发一种生物偶联反应,以使信号蛋白可逆和可重复地束缚在水凝胶中,这样就可以按需释放用过的蛋白质,并根据需要用新鲜蛋白质代替。具体而言,我们设计了一种烯丙基硫醚链转移剂,该剂可使信号蛋白与水凝胶发生可逆的,光介导的硫醇-烯生物缀合。在将硫醇化的蛋白质添加到烯丙基硫醚部分上时,释放了之前束缚的蛋白质,并且“烯”官能团被再生。使用这种方法,我们证明可以通过硫醇-烯反应在水凝胶中实现蛋白质图案化,然后可以通过随后的PEG硫醇-烯反应释放出图案化的蛋白质。重要的是,该过程可通过多次重复来重复,并以生理相关的信号蛋白浓度进行。最后,我们证明了整个信号蛋白可以在细胞存在的情况下被模式化和释放,并且细胞以空间保真度响应其表现。综合起来,这些数据代表了方法学的第一个例子,该方法能够实现可逆和可重复的模式化以及从水凝胶中释放信号蛋白。
更新日期:2018-07-12
中文翻译:

可逆和可重复的硫醇-烯生物缀合物,用于水凝胶中信号蛋白的动态模式分析。
生物分子功能化的水凝胶已成为有价值的细胞培养平台,以概括细胞外生态位的机械和生化特性。用生物分子对水凝胶进行功能化的典型策略包括将它们直接束缚在水凝胶骨架上,从而产生静态物质。因此,该方法不能捕获生物分子组成在生物过程中发生的动态变化或再生医学应用可能需要的动态变化。此外,由于信号蛋白通常在细胞培养条件下稳定性差,并且随着时间的流逝失去其生物活性,这也将生物分子的范围限制为简单的肽。为此,我们寻求开发一种生物偶联反应,以使信号蛋白可逆和可重复地束缚在水凝胶中,这样就可以按需释放用过的蛋白质,并根据需要用新鲜蛋白质代替。具体而言,我们设计了一种烯丙基硫醚链转移剂,该剂可使信号蛋白与水凝胶发生可逆的,光介导的硫醇-烯生物缀合。在将硫醇化的蛋白质添加到烯丙基硫醚部分上时,释放了之前束缚的蛋白质,并且“烯”官能团被再生。使用这种方法,我们证明可以通过硫醇-烯反应在水凝胶中实现蛋白质图案化,然后可以通过随后的PEG硫醇-烯反应释放出图案化的蛋白质。重要的是,该过程可通过多次重复来重复,并以生理相关的信号蛋白浓度进行。最后,我们证明了整个信号蛋白可以在细胞存在的情况下被模式化和释放,并且细胞以空间保真度响应其表现。综合起来,这些数据代表了方法学的第一个例子,该方法能够实现可逆和可重复的模式化以及从水凝胶中释放信号蛋白。











































 京公网安备 11010802027423号
京公网安备 11010802027423号