当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insight through Irreversible Inhibition: DNA Polymerase θ Uses a Common Active Site for Polymerase and Lyase Activities
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-12 , DOI: 10.1021/jacs.8b04158 Daniel J Laverty 1 , Ifor P Mortimer 1 , Marc M Greenberg 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-12 , DOI: 10.1021/jacs.8b04158 Daniel J Laverty 1 , Ifor P Mortimer 1 , Marc M Greenberg 1
Affiliation
|
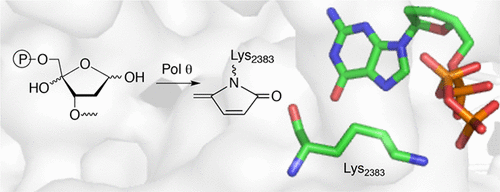
DNA polymerase θ (Pol θ) is a multifunctional enzyme. It is nonessential in normal cells, but its upregulation in cancer cells correlates with cellular resistance to oxidative damage and poor prognosis. Pol θ possesses polymerase activity and poorly characterized lyase activity. We examined the Pol θ lyase activity on various abasic sites and determined that the enzyme is inactivated upon attempted removal of the oxidized abasic site commonly associated with C4'-oxidation (pC4-AP). Covalent modification of Pol θ by the DNA lesion enabled determination of the primary nucleophile (Lys2383) responsible for Schiff base formation in the lyase reaction. Unlike some other base excision repair polymerases, Pol θ uses a single active site for polymerase and lyase activity. Mutation of Lys2383 significantly reduces both enzyme activities but not DNA binding. Demonstration that Lys2383 is required for polymerase and lyase activities indicates that this residue is an Achilles heel for Pol θ and suggests a path forward for designing inhibitors of this attractive anticancer target.
中文翻译:

通过不可逆抑制的机制洞察:DNA 聚合酶 θ 使用聚合酶和裂解酶活性的共同活性位点
DNA聚合酶θ (Pol θ) 是一种多功能酶。它在正常细胞中不是必需的,但它在癌细胞中的上调与细胞对氧化损伤的抵抗力和不良预后相关。 Pol θ 具有聚合酶活性和尚未表征的裂解酶活性。我们检查了各个脱碱基位点上的 Pol θ 裂解酶活性,并确定该酶在尝试去除通常与 C4'-氧化 (pC4-AP) 相关的氧化脱碱基位点时失活。 DNA 损伤对 Pol θ 进行共价修饰,能够确定裂解酶反应中负责希夫碱形成的主要亲核试剂 (Lys2383)。与其他一些碱基切除修复聚合酶不同,Pol θ 使用单个活性位点进行聚合酶和裂解酶活性。 Lys2383 的突变显着降低了两种酶的活性,但不降低 DNA 结合。 Lys2383 是聚合酶和裂解酶活性所必需的证明表明该残基是 Pol θ 的致命弱点,并为设计这一有吸引力的抗癌靶标的抑制剂提供了一条前进的道路。
更新日期:2018-07-12
中文翻译:

通过不可逆抑制的机制洞察:DNA 聚合酶 θ 使用聚合酶和裂解酶活性的共同活性位点
DNA聚合酶θ (Pol θ) 是一种多功能酶。它在正常细胞中不是必需的,但它在癌细胞中的上调与细胞对氧化损伤的抵抗力和不良预后相关。 Pol θ 具有聚合酶活性和尚未表征的裂解酶活性。我们检查了各个脱碱基位点上的 Pol θ 裂解酶活性,并确定该酶在尝试去除通常与 C4'-氧化 (pC4-AP) 相关的氧化脱碱基位点时失活。 DNA 损伤对 Pol θ 进行共价修饰,能够确定裂解酶反应中负责希夫碱形成的主要亲核试剂 (Lys2383)。与其他一些碱基切除修复聚合酶不同,Pol θ 使用单个活性位点进行聚合酶和裂解酶活性。 Lys2383 的突变显着降低了两种酶的活性,但不降低 DNA 结合。 Lys2383 是聚合酶和裂解酶活性所必需的证明表明该残基是 Pol θ 的致命弱点,并为设计这一有吸引力的抗癌靶标的抑制剂提供了一条前进的道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号