当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Ruthenium-Catalyzed Benzocyclobutenone–Ketol Cycloaddition: Merging C–C Bond Activation and Transfer Hydrogenative Coupling for Type II Polyketide Construction
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-11 , DOI: 10.1021/jacs.8b05724 Brett R Ambler 1 , Ben W H Turnbull 1 , Sankar Rao Suravarapu 1 , Maulen M Uteuliyev 1 , Nancy O Huynh 1 , Michael J Krische 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-11 , DOI: 10.1021/jacs.8b05724 Brett R Ambler 1 , Ben W H Turnbull 1 , Sankar Rao Suravarapu 1 , Maulen M Uteuliyev 1 , Nancy O Huynh 1 , Michael J Krische 1
Affiliation
|
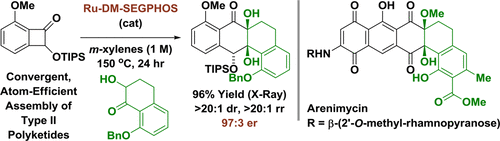
The first enantioselective intermolecular metal-catalyzed cycloadditions of benzocyclobutenones via C-C bond oxidative addition are described. In the presence of a ruthenium(0) complex modified by ( R)-DM-SEGPHOS, tetralone-derived ketols and benzocyclobutenones combine to form cycloadducts with complete regio- and diastereoselectivity and high enantioselectivity. Using this method, the "bay region" substructure of the angucycline natural product arenimycin was prepared.
中文翻译:

对映选择性钌催化苯并环丁烯酮-酮醇环加成:合并 C-C 键活化和转移氢化偶联用于 II 型聚酮化合物结构
描述了苯并环丁烯酮通过 CC 键氧化加成的第一个对映选择性分子间金属催化环加成。在 (R)-DM-SEGPHOS 修饰的钌 (0) 络合物存在下,四氢萘酮衍生的酮醇和苯并环丁烯酮结合形成具有完全区域和非对映选择性以及高对映选择性的环加合物。利用该方法制备了安古环素天然产物沙霉素的“湾区”亚结构。
更新日期:2018-07-11
中文翻译:

对映选择性钌催化苯并环丁烯酮-酮醇环加成:合并 C-C 键活化和转移氢化偶联用于 II 型聚酮化合物结构
描述了苯并环丁烯酮通过 CC 键氧化加成的第一个对映选择性分子间金属催化环加成。在 (R)-DM-SEGPHOS 修饰的钌 (0) 络合物存在下,四氢萘酮衍生的酮醇和苯并环丁烯酮结合形成具有完全区域和非对映选择性以及高对映选择性的环加合物。利用该方法制备了安古环素天然产物沙霉素的“湾区”亚结构。









































 京公网安备 11010802027423号
京公网安备 11010802027423号