当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydroamination versus Allylic Amination in Iridium-Catalyzed Reactions of Allylic Acetates with Amines: 1,3-Aminoalcohols via Ester-Directed Regioselectivity
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-10 , DOI: 10.1021/jacs.8b05683 Seung Wook Kim 1 , Thomas Wurm 1 , Gilmar A. Brito 1 , Woo-Ok Jung 1 , Jason R. Zbieg 2 , Craig E. Stivala 2 , Michael J. Krische 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-10 , DOI: 10.1021/jacs.8b05683 Seung Wook Kim 1 , Thomas Wurm 1 , Gilmar A. Brito 1 , Woo-Ok Jung 1 , Jason R. Zbieg 2 , Craig E. Stivala 2 , Michael J. Krische 1
Affiliation
|
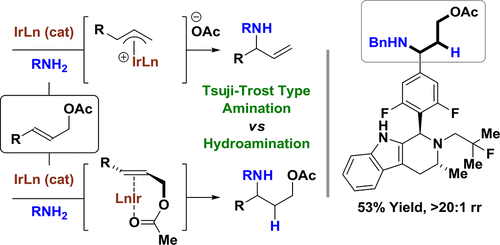
In the presence of a neutral dppf-modified iridium catalyst and Cs2CO3, linear allylic acetates react with primary amines to form products of hydroamination with complete 1,3-regioselectivity. The collective data, including deuterium labeling studies, corroborate a catalytic mechanism involving rapid, reversible acetate-directed aminoiridation with inner-sphere/outer-sphere crossover followed by turnover-limiting proto-demetalation mediated by amine.
中文翻译:

烯丙基乙酸酯与胺的铱催化反应中的氢化胺化与烯丙基胺化:1,3-氨基醇通过酯定向区域选择性
在中性 dppf 改性的铱催化剂和 Cs2CO3 存在下,线性烯丙基乙酸酯与伯胺反应形成具有完全 1,3-区域选择性的加氢胺化产物。包括氘标记研究在内的集体数据证实了一种催化机制,该机制涉及快速、可逆的醋酸盐导向氨基环化,内球/外球交叉,然后是由胺介导的限制营业额的原脱金属。
更新日期:2018-07-10
中文翻译:

烯丙基乙酸酯与胺的铱催化反应中的氢化胺化与烯丙基胺化:1,3-氨基醇通过酯定向区域选择性
在中性 dppf 改性的铱催化剂和 Cs2CO3 存在下,线性烯丙基乙酸酯与伯胺反应形成具有完全 1,3-区域选择性的加氢胺化产物。包括氘标记研究在内的集体数据证实了一种催化机制,该机制涉及快速、可逆的醋酸盐导向氨基环化,内球/外球交叉,然后是由胺介导的限制营业额的原脱金属。











































 京公网安备 11010802027423号
京公网安备 11010802027423号