当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu-Catalyzed Aerobic Oxidative N–N Coupling of Carbazoles and Diarylamines Including Selective Cross-Coupling
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-10 , DOI: 10.1021/jacs.8b05245 Michael C Ryan 1 , Joseph R Martinelli 2 , Shannon S Stahl 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-10 , DOI: 10.1021/jacs.8b05245 Michael C Ryan 1 , Joseph R Martinelli 2 , Shannon S Stahl 1
Affiliation
|
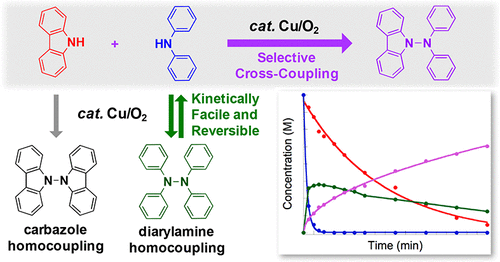
A Cu-catalyzed method has been identified for aerobic oxidative dimerization of carbazoles and diarylamines to the corresponding N-N coupled bicarbazoles and tetraarylhydrazines. The reactions proceed under mild conditions (1 atm O2, 60-80 °C) with a catalyst composed of CuBr·dimethylsulfide and N, N-dimethylaminopyridine. Reactions between carbazole and diarylamines show unusually selective cross-coupling, even with a 1:1 ratio of the two substrates. This behavior was found to arise from reversible formation of the tetraarylhydrazine. Formation of this species is kinetically favored, but cleavage of the N-N bond under the reaction conditions leads to selective formation of the thermodynamically favored cross-coupling product.
中文翻译:

铜催化咔唑和二芳胺的有氧氧化 N-N 偶联,包括选择性交叉偶联
已经确定了一种铜催化方法,用于将咔唑和二芳基胺有氧氧化二聚成相应的 NN 偶联联咔唑和四芳基肼。该反应在温和条件(1 atm O2,60-80 °C)下进行,催化剂由 CuBr·二甲基硫醚和 N,N-二甲基氨基吡啶组成。咔唑和二芳基胺之间的反应显示出异常选择性的交叉偶联,即使两种底物的比例为 1:1。发现这种行为是由四芳基肼的可逆形成引起的。该物质的形成在动力学上是有利的,但反应条件下 NN 键的断裂会导致热力学上有利的交叉偶联产物的选择性形成。
更新日期:2018-07-10
中文翻译:

铜催化咔唑和二芳胺的有氧氧化 N-N 偶联,包括选择性交叉偶联
已经确定了一种铜催化方法,用于将咔唑和二芳基胺有氧氧化二聚成相应的 NN 偶联联咔唑和四芳基肼。该反应在温和条件(1 atm O2,60-80 °C)下进行,催化剂由 CuBr·二甲基硫醚和 N,N-二甲基氨基吡啶组成。咔唑和二芳基胺之间的反应显示出异常选择性的交叉偶联,即使两种底物的比例为 1:1。发现这种行为是由四芳基肼的可逆形成引起的。该物质的形成在动力学上是有利的,但反应条件下 NN 键的断裂会导致热力学上有利的交叉偶联产物的选择性形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号