Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structures of human Patched and its complex with native palmitoylated sonic hedgehog
Nature ( IF 64.8 ) Pub Date : 2018-07-11 , DOI: 10.1038/s41586-018-0308-7 Xiaofeng Qi , Philip Schmiege , Elias Coutavas , Jiawei Wang , Xiaochun Li
Nature ( IF 64.8 ) Pub Date : 2018-07-11 , DOI: 10.1038/s41586-018-0308-7 Xiaofeng Qi , Philip Schmiege , Elias Coutavas , Jiawei Wang , Xiaochun Li
|
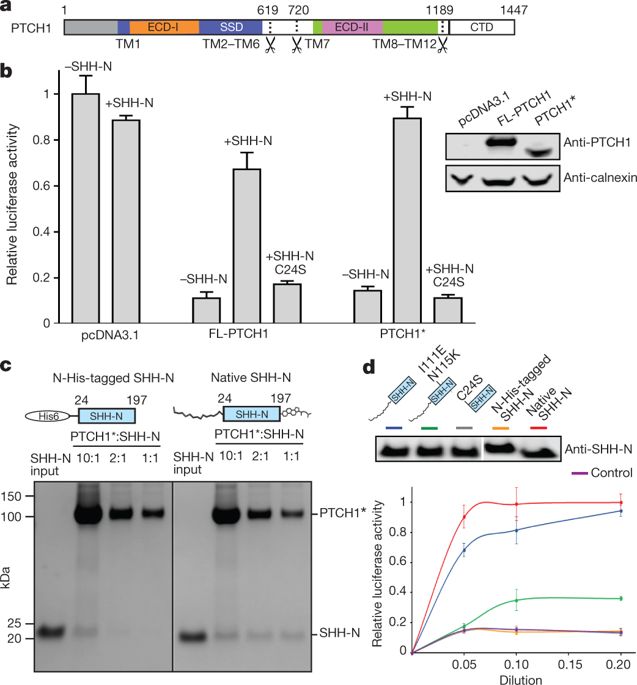
Hedgehog (HH) signalling governs embryogenesis and adult tissue homeostasis in mammals and other multicellular organisms1–3. Whereas deficient HH signalling leads to birth defects, unrestrained HH signalling is implicated in human cancers2,4–6. N-terminally palmitoylated HH releases the repression of Patched to the oncoprotein smoothened (SMO); however, the mechanism by which HH recognizes Patched is unclear. Here we report cryo-electron microscopy structures of human patched 1 (PTCH1) alone and in complex with the N-terminal domain of ‘native’ sonic hedgehog (native SHH-N has both a C-terminal cholesterol and an N-terminal fatty-acid modification), at resolutions of 3.5 Å and 3.8 Å, respectively. The structure of PTCH1 has internal two-fold pseudosymmetry in the transmembrane core, which features a sterol-sensing domain and two homologous extracellular domains, resembling the architecture of Niemann–Pick C1 (NPC1) protein7. The palmitoylated N terminus of SHH-N inserts into a cavity between the extracellular domains of PTCH1 and dominates the PTCH1–SHH-N interface, which is distinct from that reported for SHH-N co-receptors8. Our biochemical assays show that SHH-N may use another interface, one that is required for its co-receptor binding, to recruit PTCH1 in the absence of a covalently attached palmitate. Our work provides atomic insights into the recognition of the N-terminal domain of HH (HH-N) by PTCH1, offers a structural basis for cooperative binding of HH-N to various receptors and serves as a molecular framework for HH signalling and its malfunction in disease.High-resolution structures of the human plasma membrane protein patched 1 alone and in complex with the native form of the ligand sonic hedgehog are determined.
中文翻译:

人类Patched及其与天然棕榈酰化声波刺猬复合物的结构
Hedgehog (HH) 信号传导控制哺乳动物和其他多细胞生物的胚胎发生和成体组织稳态1-3。虽然缺乏 HH 信号会导致出生缺陷,但不受限制的 HH 信号与人类癌症有关 2,4-6。N-末端棕榈酰化 HH 释放 Patched 对癌蛋白平滑 (SMO) 的抑制;然而,HH 识别 Patched 的机制尚不清楚。在这里,我们报告了单独的人类修补程序 1 (PTCH1) 以及与“天然”声波刺猬的 N 端结构域(天然 SHH-N 具有 C 端胆固醇和 N 端脂肪-酸修饰),分辨率分别为 3.5 Å 和 3.8 Å。PTCH1的结构在跨膜核中具有内部双重伪对称性,它具有一个甾醇感应域和两个同源的细胞外域,类似于 Niemann-Pick C1 (NPC1) 蛋白的结构7。SHH-N 的棕榈酰化 N 末端插入 PTCH1 细胞外结构域之间的空腔中,并支配 PTCH1-SHH-N 界面,这与报道的 SHH-N 共受体 8 不同。我们的生化分析表明,SHH-N 可能使用另一种界面,这是其共受体结合所需的界面,在没有共价连接的棕榈酸酯的情况下募集 PTCH1。我们的工作为 PTCH1 识别 HH (HH-N) 的 N 端结构域提供了原子见解,为 HH-N 与各种受体的协同结合提供了结构基础,并作为 HH 信号传导及其故障的分子框架在疾病中。
更新日期:2018-07-11
中文翻译:

人类Patched及其与天然棕榈酰化声波刺猬复合物的结构
Hedgehog (HH) 信号传导控制哺乳动物和其他多细胞生物的胚胎发生和成体组织稳态1-3。虽然缺乏 HH 信号会导致出生缺陷,但不受限制的 HH 信号与人类癌症有关 2,4-6。N-末端棕榈酰化 HH 释放 Patched 对癌蛋白平滑 (SMO) 的抑制;然而,HH 识别 Patched 的机制尚不清楚。在这里,我们报告了单独的人类修补程序 1 (PTCH1) 以及与“天然”声波刺猬的 N 端结构域(天然 SHH-N 具有 C 端胆固醇和 N 端脂肪-酸修饰),分辨率分别为 3.5 Å 和 3.8 Å。PTCH1的结构在跨膜核中具有内部双重伪对称性,它具有一个甾醇感应域和两个同源的细胞外域,类似于 Niemann-Pick C1 (NPC1) 蛋白的结构7。SHH-N 的棕榈酰化 N 末端插入 PTCH1 细胞外结构域之间的空腔中,并支配 PTCH1-SHH-N 界面,这与报道的 SHH-N 共受体 8 不同。我们的生化分析表明,SHH-N 可能使用另一种界面,这是其共受体结合所需的界面,在没有共价连接的棕榈酸酯的情况下募集 PTCH1。我们的工作为 PTCH1 识别 HH (HH-N) 的 N 端结构域提供了原子见解,为 HH-N 与各种受体的协同结合提供了结构基础,并作为 HH 信号传导及其故障的分子框架在疾病中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号