当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Reactivity of o‐Enoyl Arylisocyanides: Access to Phenanthridine‐8‐Carboxylate Derivatives
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-08-02 , DOI: 10.1002/adsc.201800606 Jinxiong Cai 1, 2 , Zhongyan Hu 1, 2 , Yifei Li 2 , Jun Liu 2 , Xianxiu Xu 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-08-02 , DOI: 10.1002/adsc.201800606 Jinxiong Cai 1, 2 , Zhongyan Hu 1, 2 , Yifei Li 2 , Jun Liu 2 , Xianxiu Xu 1
Affiliation
|
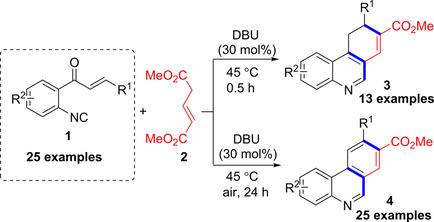
Various o‐enoyl arylisocyanides were prepared from readily available reactants and found to undergo a double annulation with glutaconate to provide straightforward access to phenanthridine‐8‐carboxylates and hydrophenanthridine‐8‐carboxylates under mild aerobic conditions. In this domino transformation, two rings and three bonds were successively created. A mechanism involving tandem [3+3]‐annulation/intramolecular cyclization/ demethoxycarbonylation/aerobic oxidative aromatization sequence was proposed.
中文翻译:

邻烯丙基芳基异氰酸酯的合成和反应性:菲啶-8-羧酸盐衍生物的获得
从容易获得的反应物中制备了各种邻烯基芳基异氰酸酯,发现它们与戊二酸进行了双环化反应,从而可以在温和的好氧条件下直接获得菲啶-8-羧酸盐和氢化菲啶-8-羧酸盐。在该多米诺骨牌变换中,两个环和三个键被连续创建。提出了一种机制,涉及串联[3 + 3]-环化/分子内环化/脱甲氧基羰基化/好氧氧化芳构化顺序。
更新日期:2018-08-02
中文翻译:

邻烯丙基芳基异氰酸酯的合成和反应性:菲啶-8-羧酸盐衍生物的获得
从容易获得的反应物中制备了各种邻烯基芳基异氰酸酯,发现它们与戊二酸进行了双环化反应,从而可以在温和的好氧条件下直接获得菲啶-8-羧酸盐和氢化菲啶-8-羧酸盐。在该多米诺骨牌变换中,两个环和三个键被连续创建。提出了一种机制,涉及串联[3 + 3]-环化/分子内环化/脱甲氧基羰基化/好氧氧化芳构化顺序。











































 京公网安备 11010802027423号
京公网安备 11010802027423号