当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Synthesis of Bioactive Naturally Derived Cyclic Peptides Containing Ene‐Like Rigidifying Motifs
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-10-18 , DOI: 10.1002/chem.201802533 Luis M. De Leon Rodriguez 1 , Elyse T. Williams 2 , Margaret A. Brimble 1, 2
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-10-18 , DOI: 10.1002/chem.201802533 Luis M. De Leon Rodriguez 1 , Elyse T. Williams 2 , Margaret A. Brimble 1, 2
Affiliation
|
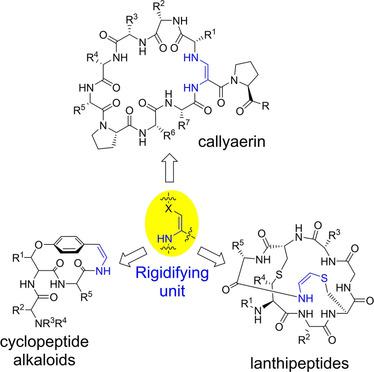
The development of synthetic methods to prepare conformationally constrained peptides and peptide–polyketide hybrids remain an important chemical challenge. It is known that structural rigidity correlates with the specificity, bioactivity, and stability of these peptide systems, thus rigid systems are particularly attractive leads for development of potent biopharmaceuticals. Herein we provide an overview of recent developments in the syntheses of naturally derived constrained peptides and peptide–polyketide hybrids, with a particular emphasis on those systems containing an ene‐like bond.
中文翻译:

化学合成的生物活性天然衍生的环肽,具有类似刚性的基序。
制备构象受限的肽和肽-聚酮杂化物的合成方法的发展仍然是一个重要的化学挑战。已知结构刚性与这些肽系统的特异性,生物活性和稳定性相关,因此刚性系统是开发有效生物药物的特别有吸引力的线索。本文中,我们概述了自然衍生的受约束肽和肽-聚酮化合物杂化物的合成的最新进展,特别强调了那些包含烯键的体系。
更新日期:2018-10-18
中文翻译:

化学合成的生物活性天然衍生的环肽,具有类似刚性的基序。
制备构象受限的肽和肽-聚酮杂化物的合成方法的发展仍然是一个重要的化学挑战。已知结构刚性与这些肽系统的特异性,生物活性和稳定性相关,因此刚性系统是开发有效生物药物的特别有吸引力的线索。本文中,我们概述了自然衍生的受约束肽和肽-聚酮化合物杂化物的合成的最新进展,特别强调了那些包含烯键的体系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号