当前位置:
X-MOL 学术
›
Food Funct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a microparticulate system containing Brazilian propolis by-product and gelatine for ascorbic acid delivery: evaluation of intestinal cell viability and radical scavenging activity
Food & Function ( IF 5.1 ) Pub Date : 2018-07-11 , DOI: 10.1039/c8fo00863a Lizziane Maria Belloto de Francisco 1, 2, 3, 4, 5 , Diana Pinto 6, 7, 8, 9, 10 , Hélen Cássia Rosseto 1, 2, 3, 4, 5 , Lucas de Alcântara Sica de Toledo 1, 2, 3, 4, 5 , Rafaela Said dos Santos 1, 2, 3, 4, 5 , Paulo Costa 8, 9, 10, 11, 12 , Francisca Rodrigues 6, 7, 8, 9, 10 , M. Beatriz P. P. Oliveira 6, 7, 8, 9, 10 , Bruno Sarmento 9, 13, 14, 15, 16 , Marcos Luciano Bruschi 1, 2, 3, 4, 5
Food & Function ( IF 5.1 ) Pub Date : 2018-07-11 , DOI: 10.1039/c8fo00863a Lizziane Maria Belloto de Francisco 1, 2, 3, 4, 5 , Diana Pinto 6, 7, 8, 9, 10 , Hélen Cássia Rosseto 1, 2, 3, 4, 5 , Lucas de Alcântara Sica de Toledo 1, 2, 3, 4, 5 , Rafaela Said dos Santos 1, 2, 3, 4, 5 , Paulo Costa 8, 9, 10, 11, 12 , Francisca Rodrigues 6, 7, 8, 9, 10 , M. Beatriz P. P. Oliveira 6, 7, 8, 9, 10 , Bruno Sarmento 9, 13, 14, 15, 16 , Marcos Luciano Bruschi 1, 2, 3, 4, 5
Affiliation
|
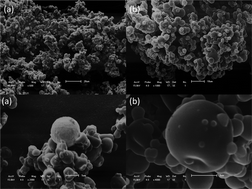
The use of propolis by-product (PBP) microparticles (MP) as delivery systems can be a promising tool to surpass drawbacks related to low stability of ascorbic acid (AA). The objective of this study was to develop and characterize MP prepared with PBP containing AA. The MP was characterized regarding morphology, particle size, polydispersity index (PDI), association efficiency (AE), drug loading (DL), infrared and Raman spectroscopy as well as antioxidant and radical scavenging activity, in vitro release, and cellular studies. MP was shown to be spherical with some agglomeration. Its particle size was 1654 ± 0.210 nm with a PDI of 0.7. The AE and DL were, respectively, 100.30 ± 2.66% and 13.16 ± 0.59. Spectroscopic studies indicated a possible interaction between the PBP and AA. 2,2-Diphenyl-1-picrylhydrazyl (DPPH˙), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) and ferric reducing antioxidant power (FRAP) assays demonstrated that the MP containing AA have an excellent antioxidant capacity as well as a considerable scavenging activity against reactive oxygen and nitrogen species. The in vitro release profile showed a slow pattern of drug release of AA from MP. Viability studies with intestinal cells revealed that MP did not present toxicity in Caco-2 and HT29-MTX. Moreover, AA could permeate Caco-2 monolayers and triple co-culture substantially at the end of 8 h, opposite to the MP. Therefore, the proposed MP formulation represents a promising platform for oral delivery of AA with a local effect on intestines.
中文翻译:

包含巴西蜂胶副产物和明胶的抗坏血酸递送微粒系统的开发:评估肠道细胞活力和自由基清除活性
蜂胶副产物(PBP)微粒(MP)作为输送系统的使用可以成为克服与抗坏血酸(AA)稳定性低有关的缺点的有前途的工具。这项研究的目的是开发和表征用含有AA的PBP制备的MP。MP在体外具有形态,粒径,多分散指数(PDI),缔合效率(AE),载药量(DL),红外和拉曼光谱以及抗氧化剂和自由基清除活性的特性释放和细胞研究。MP被证明是球形的,有一些附聚。其粒径为1654±0.210 nm,PDI为0.7。AE和DL分别为100.30±2.66%和13.16±0.59。光谱研究表明PBP和AA之间可能存在相互作用。2,2-二苯基-1-吡咯肼基(DPPH˙),2,2'-叠氮基双(3-乙基苯并噻唑啉-6-磺酸)(ABTS)和三价铁还原抗氧化能力(FRAP)分析表明MP含有AA具有出色的抗氧化能力以及对活性氧和氮物种的相当大的清除活性。在体外释放曲线显示了MP从AA释放药物的缓慢模式。对肠道细胞的生存能力研究表明,MP对Caco-2和HT29-MTX没有毒性。而且,AA基本上可以在8小时结束时渗透到Caco-2单层并进行三重共培养,这与MP相反。因此,所提出的MP制剂代表了口服给药AA的有希望的平台,其对肠道具有局部作用。
更新日期:2018-08-15
中文翻译:

包含巴西蜂胶副产物和明胶的抗坏血酸递送微粒系统的开发:评估肠道细胞活力和自由基清除活性
蜂胶副产物(PBP)微粒(MP)作为输送系统的使用可以成为克服与抗坏血酸(AA)稳定性低有关的缺点的有前途的工具。这项研究的目的是开发和表征用含有AA的PBP制备的MP。MP在体外具有形态,粒径,多分散指数(PDI),缔合效率(AE),载药量(DL),红外和拉曼光谱以及抗氧化剂和自由基清除活性的特性释放和细胞研究。MP被证明是球形的,有一些附聚。其粒径为1654±0.210 nm,PDI为0.7。AE和DL分别为100.30±2.66%和13.16±0.59。光谱研究表明PBP和AA之间可能存在相互作用。2,2-二苯基-1-吡咯肼基(DPPH˙),2,2'-叠氮基双(3-乙基苯并噻唑啉-6-磺酸)(ABTS)和三价铁还原抗氧化能力(FRAP)分析表明MP含有AA具有出色的抗氧化能力以及对活性氧和氮物种的相当大的清除活性。在体外释放曲线显示了MP从AA释放药物的缓慢模式。对肠道细胞的生存能力研究表明,MP对Caco-2和HT29-MTX没有毒性。而且,AA基本上可以在8小时结束时渗透到Caco-2单层并进行三重共培养,这与MP相反。因此,所提出的MP制剂代表了口服给药AA的有希望的平台,其对肠道具有局部作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号