当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Generation and Alkylation of α-Carbamyl Radicals via Organic Photoredox Catalysis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-09 , DOI: 10.1021/jacs.8b04890 Joshua B. McManus 1 , Nicholas P. R. Onuska 1 , David A. Nicewicz 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-09 , DOI: 10.1021/jacs.8b04890 Joshua B. McManus 1 , Nicholas P. R. Onuska 1 , David A. Nicewicz 1
Affiliation
|
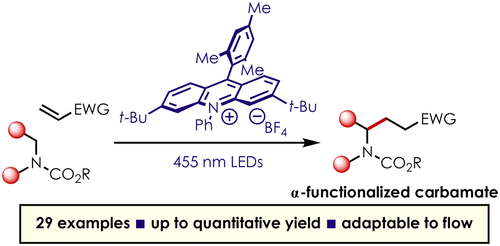
Strategies for the direct C-H functionalization of amines are valuable as these compounds comprise a number of pharmaceuticals, agrochemicals and natural products. This work describes a novel method for the C-H functionalization of carbamate-protected secondary amines via α-carbamyl radicals generated using photoredox catalysis. The use of the highly oxidizing, organic acridinium photoredox catalyst allows for direct oxidation of carbamate-protected amines with high redox potentials to give the corresponding carbamyl cation radical. Following deprotonation, the resultant open-shell species can be intercepted by a variety of Michael acceptors to give elaborate α-functionalized secondary amines. The reaction proceeds under mild conditions without the requirement of exogenous redox mediators or substrate prefunctionalization. Additionally, we were able to showcase the utility of this methodology through the enantioselective synthesis of the indolizidine alkaloid, (+)-monomorine I.
中文翻译:

通过有机光氧化还原催化生成和烷基化 α-氨基甲酰基自由基
胺的直接 CH 官能化策略很有价值,因为这些化合物包括许多药物、农用化学品和天然产品。这项工作描述了一种通过使用光氧化还原催化产生的 α-氨基甲酰基自由基对氨基甲酸酯保护的仲胺进行 CH 官能化的新方法。使用高氧化性有机吖啶光氧化还原催化剂可以直接氧化具有高氧化还原电位的氨基甲酸酯保护的胺,得到相应的氨基甲酰基阳离子自由基。去质子化后,所得的开壳物质可以被各种迈克尔受体截获,得到精心设计的 α-官能化仲胺。该反应在温和的条件下进行,不需要外源氧化还原介质或底物预官能化。此外,
更新日期:2018-07-09
中文翻译:

通过有机光氧化还原催化生成和烷基化 α-氨基甲酰基自由基
胺的直接 CH 官能化策略很有价值,因为这些化合物包括许多药物、农用化学品和天然产品。这项工作描述了一种通过使用光氧化还原催化产生的 α-氨基甲酰基自由基对氨基甲酸酯保护的仲胺进行 CH 官能化的新方法。使用高氧化性有机吖啶光氧化还原催化剂可以直接氧化具有高氧化还原电位的氨基甲酸酯保护的胺,得到相应的氨基甲酰基阳离子自由基。去质子化后,所得的开壳物质可以被各种迈克尔受体截获,得到精心设计的 α-官能化仲胺。该反应在温和的条件下进行,不需要外源氧化还原介质或底物预官能化。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号