当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dried-Blood-Spot Technique to Monitor Direct Oral Anticoagulants: Clinical Validation of a UPLC–MS/MS-Based Assay
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-07-09 00:00:00 , DOI: 10.1021/acs.analchem.8b02046 Kathrin I. Foerster 1 , Andrea Huppertz 1 , Andreas D. Meid 1 , Oliver J. Müller 2 , Timolaos Rizos 3 , Lisa Tilemann 2 , Walter E. Haefeli 1 , Jürgen Burhenne 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-07-09 00:00:00 , DOI: 10.1021/acs.analchem.8b02046 Kathrin I. Foerster 1 , Andrea Huppertz 1 , Andreas D. Meid 1 , Oliver J. Müller 2 , Timolaos Rizos 3 , Lisa Tilemann 2 , Walter E. Haefeli 1 , Jürgen Burhenne 1
Affiliation
|
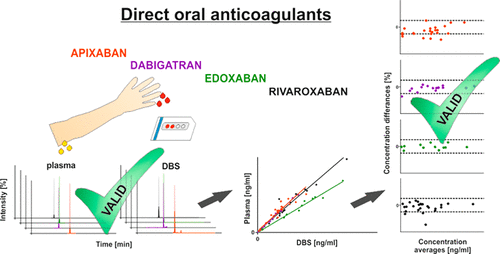
Plasma concentrations of direct oral anticoagulants (DOACs) vary largely between individuals, and they correlate well with desired and adverse outcomes. Although regular concentration monitoring of DOACs is not recommended, information on DOAC exposure could be useful in situations when multiple DOAC-clearance pathways are impaired or nonadherence is suspected. Self-sampling techniques, like the use of dried-blood spots (DBSs), would be particularly useful because they enable the collection of information in ambulatory patients at relevant points in time of the dosing interval (e.g., trough). We developed and validated a DBS-based assay to quantify all currently marketed DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) in a single ultraperformance-liquid-chromatography–tandem-mass-spectrometry assay. It fulfilled all validation standards within a hematocrit range of 0.33–0.65 and was linear over the calibration ranges of 2.5–750 ng/mL (apixaban and rivaroxaban), 4.4–750 ng/mL (dabigatran), and 9.3–750 ng/mL (edoxaban). Only minor ion suppression (matrix effect ≤13%) was present, inter- and intra-assay precision was ≤13%, and inter- and intra-assay accuracies ranged between 88 and 110%. All DOACs were stable in DBSs up to 52 days at room temperature, if the DBSs were protected from light and humidity. The correlation between (whole blood) DBS and plasma concentrations was assessed in 33 patients under regular DOAC therapy. Deming-regression coefficients between simultaneously collected capillary DBSs and plasma samples were used to predict plasma concentrations from DBSs. Bland–Altman plots revealed a strong agreement between predicted and observed plasma concentrations, thus confirming the suitability of DBSs for DOAC monitoring as an important step toward the important aim of self-sampling at home.
中文翻译:

干血斑技术监测直接口服抗凝剂:基于UPLC–MS / MS的检测方法的临床验证
个体之间直接口服抗凝剂(DOAC)的血浆浓度差异很大,并且与预期和不良后果之间存在良好的相关性。尽管不建议定期对DOAC进行浓度监测,但在多个DOAC清除途径受损或怀疑不依从的情况下,有关DOAC暴露的信息可能会很有用。自采样技术(如使用干血斑(DBS))将特别有用,因为它们可以在给药间隔的相关时间点(例如,谷底)收集非卧床患者的信息。我们开发并验证了基于DBS的测定法,以单次超高效液相色谱-串联质谱测定法对当前所有市售的DOAC(阿哌沙班,达比加群,依多沙班和利伐沙班)进行定量。它在血细胞比容范围为0.33-0.65的范围内满足所有验证标准,并且在2.5-750 ng / mL(阿哌沙班和利伐沙班),4.4-750 ng / mL(达比加群)和9.3-750 ng / mL的校准范围内呈线性(edoxaban)。仅存在较小的离子抑制(基质效应≤13%),批间和批内精密度≤13%,批间和批内准确度在88%至110%之间。如果保护DBS免受光照和潮湿,则所有DOAC在DBS中在室温下最多可稳定52天。在常规DOAC治疗下对33例患者(全血)DBS与血浆浓度之间的相关性进行了评估。同时收集的毛细管DBS和血浆样品之间的戴明回归系数用于预测DBS的血浆浓度。
更新日期:2018-07-09
中文翻译:

干血斑技术监测直接口服抗凝剂:基于UPLC–MS / MS的检测方法的临床验证
个体之间直接口服抗凝剂(DOAC)的血浆浓度差异很大,并且与预期和不良后果之间存在良好的相关性。尽管不建议定期对DOAC进行浓度监测,但在多个DOAC清除途径受损或怀疑不依从的情况下,有关DOAC暴露的信息可能会很有用。自采样技术(如使用干血斑(DBS))将特别有用,因为它们可以在给药间隔的相关时间点(例如,谷底)收集非卧床患者的信息。我们开发并验证了基于DBS的测定法,以单次超高效液相色谱-串联质谱测定法对当前所有市售的DOAC(阿哌沙班,达比加群,依多沙班和利伐沙班)进行定量。它在血细胞比容范围为0.33-0.65的范围内满足所有验证标准,并且在2.5-750 ng / mL(阿哌沙班和利伐沙班),4.4-750 ng / mL(达比加群)和9.3-750 ng / mL的校准范围内呈线性(edoxaban)。仅存在较小的离子抑制(基质效应≤13%),批间和批内精密度≤13%,批间和批内准确度在88%至110%之间。如果保护DBS免受光照和潮湿,则所有DOAC在DBS中在室温下最多可稳定52天。在常规DOAC治疗下对33例患者(全血)DBS与血浆浓度之间的相关性进行了评估。同时收集的毛细管DBS和血浆样品之间的戴明回归系数用于预测DBS的血浆浓度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号