当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Binding indirect greenhouse gases OCS and CS2 by nitrogen heterocyclic carbenes (NHCs)†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-07-09 00:00:00 , DOI: 10.1039/c8cp03217c M. Merced Montero-Campillo 1, 2, 3, 4, 5 , Ibon Alkorta 5, 6, 7 , José Elguero 5, 6, 7
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-07-09 00:00:00 , DOI: 10.1039/c8cp03217c M. Merced Montero-Campillo 1, 2, 3, 4, 5 , Ibon Alkorta 5, 6, 7 , José Elguero 5, 6, 7
Affiliation
|
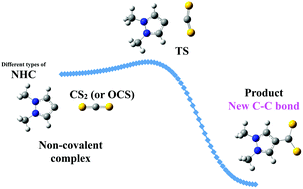
Carbon disulfide (CS2) and carbonyl sulfide (OCS) are indirect greenhouse gases that can be effectively trapped by classical, abnormal and remote nitrogen heterocyclic carbenes (NHCs), according to high level ab initio calculations. The process is described through a reaction profile involving two minima, a non-covalent complex and a covalently bound product, connected by a single transition state. Both CS2 and OCS react towards NHCs in a similar way, forming a new C–C bond and leading to very stable products with feasible barriers in many cases, although they vary significantly depending on the NHC structure. The barriers are larger than those reported for CO2, oscillating from barrierless processes up to a maximum of 57.9 kJ mol−1, whereas the products are more stabilized than those incorporating CO2. The lowest barriers for the CS2 + NHC reactions correspond to the largest C–C distances in the products, unlike the CO2 case. Remarkably, the most favored reactions, which are those involving a remote NHC, do not exhibit the highest interaction energies at the TS, but low distortion energy values of the OCS/CS2 moieties. The decomposition of the interaction energy allowed to confirmed that in fact the remote carbene is the less favored one in terms of the electrostatic and exchange terms. Substitution in CO2 of O by a more polarizable atom such as S have a great influence on the balance between the steric plus orbital interaction and the kinetic energy terms, thus making the products between NHCs and OCS/CS2 more stable. Both OCS and CS2 become better charge acceptors than CO2 on going from the starting complexes to the products.
中文翻译:

氮杂环卡宾(NHC) 结合间接温室气体OCS和CS 2 †
根据高级从头算的计算,二硫化碳(CS 2)和羰基硫化物(OCS)是间接的温室气体,可以被经典的,异常的和偏远的氮杂环卡宾(NHC)有效地捕集。通过反应概况描述了该过程,该反应概况涉及通过单一过渡态连接的两个极小值(非共价复合物和共价结合的产物)。CS 2和OCS都以类似的方式对NHC做出反应,形成一个新的C-C键,并在很多情况下形成具有可行壁垒的非常稳定的产物,尽管它们的变化取决于NHC的结构。势垒比报告的CO 2势垒更大,由无障碍过程产生,最高可达57.9 kJ mol。-1,而产物比掺入CO 2的产物更稳定。与CO 2情况不同,CS 2 + NHC反应的最低障碍对应于产品中最大的C–C距离。值得注意的是,最受青睐的反应是涉及远程NHC的反应,在TS处未显示出最高的相互作用能,但OCS / CS 2部分的畸变能值较低。相互作用能的分解可以证实,事实上,就静电和交换而言,远距离卡宾是较不受欢迎的。替代CO 2O的极性更强的原子(例如S)对空间加轨道相互作用与动能项之间的平衡有很大影响,因此使NHC和OCS / CS 2之间的产物更稳定。这两个OCS和CS 2成为更好的电荷受体比CO 2从起始复合物的产品去。
更新日期:2018-07-09
中文翻译:

氮杂环卡宾(NHC) 结合间接温室气体OCS和CS 2 †
根据高级从头算的计算,二硫化碳(CS 2)和羰基硫化物(OCS)是间接的温室气体,可以被经典的,异常的和偏远的氮杂环卡宾(NHC)有效地捕集。通过反应概况描述了该过程,该反应概况涉及通过单一过渡态连接的两个极小值(非共价复合物和共价结合的产物)。CS 2和OCS都以类似的方式对NHC做出反应,形成一个新的C-C键,并在很多情况下形成具有可行壁垒的非常稳定的产物,尽管它们的变化取决于NHC的结构。势垒比报告的CO 2势垒更大,由无障碍过程产生,最高可达57.9 kJ mol。-1,而产物比掺入CO 2的产物更稳定。与CO 2情况不同,CS 2 + NHC反应的最低障碍对应于产品中最大的C–C距离。值得注意的是,最受青睐的反应是涉及远程NHC的反应,在TS处未显示出最高的相互作用能,但OCS / CS 2部分的畸变能值较低。相互作用能的分解可以证实,事实上,就静电和交换而言,远距离卡宾是较不受欢迎的。替代CO 2O的极性更强的原子(例如S)对空间加轨道相互作用与动能项之间的平衡有很大影响,因此使NHC和OCS / CS 2之间的产物更稳定。这两个OCS和CS 2成为更好的电荷受体比CO 2从起始复合物的产品去。











































 京公网安备 11010802027423号
京公网安备 11010802027423号