当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel oxide–polypyrrole nanocomposite electrode materials for electrocatalytic water oxidation†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-07-09 00:00:00 , DOI: 10.1039/c7cy01949a Daniela V. Morales 1, 2, 3, 4, 5 , Catalina N. Astudillo 1, 2, 3, 4, 5 , Youssef Lattach 1, 2, 3, 4, 5 , Bruno F. Urbano 6, 7, 8, 9 , Eduardo Pereira 6, 7, 8, 9 , Bernabé L. Rivas 6, 7, 8, 9 , Josiane Arnaud 4, 5, 10, 11, 12 , Jean-Luc Putaux 1, 2, 4, 5, 13 , Selim Sirach 1, 2, 3, 4, 5 , Saioa Cobo 1, 2, 3, 4, 5 , Jean-Claude Moutet 1, 2, 3, 4, 5 , Marie-Noëlle Collomb 1, 2, 3, 4, 5 , Jérôme Fortage 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-07-09 00:00:00 , DOI: 10.1039/c7cy01949a Daniela V. Morales 1, 2, 3, 4, 5 , Catalina N. Astudillo 1, 2, 3, 4, 5 , Youssef Lattach 1, 2, 3, 4, 5 , Bruno F. Urbano 6, 7, 8, 9 , Eduardo Pereira 6, 7, 8, 9 , Bernabé L. Rivas 6, 7, 8, 9 , Josiane Arnaud 4, 5, 10, 11, 12 , Jean-Luc Putaux 1, 2, 4, 5, 13 , Selim Sirach 1, 2, 3, 4, 5 , Saioa Cobo 1, 2, 3, 4, 5 , Jean-Claude Moutet 1, 2, 3, 4, 5 , Marie-Noëlle Collomb 1, 2, 3, 4, 5 , Jérôme Fortage 1, 2, 3, 4, 5
Affiliation
|
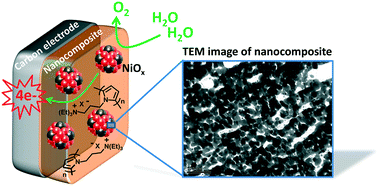
An efficient nanocomposite anode material for the oxygen evolution reaction (OER) based on nickel oxide nanoparticles entrapped in a polypyrrole matrix has been readily synthesized by an all-electrochemical procedure. Examples of anodes for the OER utilizing metallic nanoparticles dispersed in an organic polymer film are still scarce but very promising for water splitting applications. Herein, nickel metal (Ni0) nanoparticles were first electrodeposited into a cationic poly(pyrrole-ammonium) film (poly1) electropolymerized onto carbon or ITO electrodes by reduction of an anionic nickel oxalate complex in aqueous borate buffer at pH 6. The Ni0 nanoparticles were then electro-oxidized into nickel oxide (NiOx) in aqueous borate buffer at pH 9.2. The physical, chemical and electrocatalytic properties of the nanostructured poly1–NiOx material were evaluated by inductively coupled plasma mass spectroscopy (ICP-MS), atomic force microscopy (AFM) and transmission electron microscopy (TEM) coupled to various electrochemical techniques, and the results were compared with those of NiOx directly deposited on a naked electrode (without polymer) by the same electrochemical procedure. Such characterization clearly evidences the beneficial role of the poly1 matrix to generate smaller and non-agglomerated NiOx particles. Owing to the small size of NiOx nanoparticles (ca. 21 nm) and the great nanostructuration of the poly1–NiOx composite film deposited on the carbon (C) electrode surface, this material displays a very high electrocatalytic OER performance in a nearly neutral solution (pH 9.2) and even in alkaline solution (pH 14) with strong mass activities and turnover frequencies (TOFs) (1.12 A mg−1 and 0.17 s−1, respectively, at an overpotential of 0.61 V at pH 9.2, and of 1.28 A mg−1 and 0.19 s−1, respectively, at an overpotential of 0.35 V at pH 14). This performance places the C/poly1–NiOx electrode among the most active anodes based on nickel oxide reported in the literature and undoped with iron ions for the OER. In addition, when the poly1–NiOx film is deposited on a porous ITO electrode, its physisorption on the electrode surface is significantly enhanced, as shown by long-term electrocatalysis at 1.2 V vs. Ag/AgCl. Indeed, its catalytic current remains constant after several days of electrocatalysis, demonstrating the great stability of the poly1–NiOx nanocomposite; the polymer matrix maintains the integrity of the NiOx nanoparticles and precludes their corrosion. This material is thus very promising for the implementation of efficient and highly stable anodes for water oxidation.
中文翻译:

用于电催化水氧化的氧化镍-聚吡咯纳米复合电极材料†
已经通过全电化学方法容易地合成了一种有效的纳米复合阳极材料,用于基于聚吡咯基质中截留的氧化镍纳米粒子的氧释放反应(OER)。利用分散在有机聚合物膜中的金属纳米颗粒的OER阳极的例子仍然很少,但对于水分解应用非常有前途。在此,首先通过在pH值为6的硼酸盐水溶液中还原阴离子草酸镍络合物,将镍金属(Ni 0)纳米颗粒电沉积到电聚合到碳或ITO电极上的阳离子聚吡咯-铵(poly 1)膜中。然后将0个纳米粒子电氧化为氧化镍(NiO x)在pH 9.2的硼酸水溶液中缓冲。通过电感耦合等离子体质谱(ICP-MS),原子力显微镜(AFM)和透射电子显微镜(TEM)以及各种电化学技术,评估了纳米结构聚1- NiO x材料的物理,化学和电催化性能。将结果与通过相同的电化学程序直接沉积在裸电极(无聚合物)上的NiO x的结果进行了比较。这样的表征清楚地证明了poly 1基质产生较小且无团聚的NiO x颗粒的有益作用。由于NiO x纳米粒子的尺寸较小(约21 nm)和在碳(C)电极表面沉积的聚1- NiO x复合膜的良好纳米结构,这种材料在接近中性的溶液(pH 9.2)甚至在碱性溶液中均表现出很高的电催化OER性能。 pH值14)具有很强的质量活度和转换频率(TOFs)(分别在pH 9.2和0.61 V的过电势下为1.12 A mg -1和0.17 s -1以及1.28 A mg -1和0.19 s -1,分别在pH 14的0.35 V过电势下)。此性能将C / poly 1 –NiO x文献中报道的基于氧化镍的活性最高的阳极中的电极,并且在OER中未掺杂铁离子。此外,当在多孔ITO电极上沉积多1 -NiO x膜时,如在1.2 V vs. Ag / AgCl的条件下进行长期电催化所显示的,其在电极表面的物理吸附显着增强。的确,经过数天的电催化后,其催化电流保持恒定,这表明了聚1- NiO x纳米复合材料的出色稳定性。聚合物基体保持NiO x的完整性纳米颗粒并防止其腐蚀。因此,这种材料对于实现有效且高度稳定的水氧化阳极非常有前途。
更新日期:2018-07-09
中文翻译:

用于电催化水氧化的氧化镍-聚吡咯纳米复合电极材料†
已经通过全电化学方法容易地合成了一种有效的纳米复合阳极材料,用于基于聚吡咯基质中截留的氧化镍纳米粒子的氧释放反应(OER)。利用分散在有机聚合物膜中的金属纳米颗粒的OER阳极的例子仍然很少,但对于水分解应用非常有前途。在此,首先通过在pH值为6的硼酸盐水溶液中还原阴离子草酸镍络合物,将镍金属(Ni 0)纳米颗粒电沉积到电聚合到碳或ITO电极上的阳离子聚吡咯-铵(poly 1)膜中。然后将0个纳米粒子电氧化为氧化镍(NiO x)在pH 9.2的硼酸水溶液中缓冲。通过电感耦合等离子体质谱(ICP-MS),原子力显微镜(AFM)和透射电子显微镜(TEM)以及各种电化学技术,评估了纳米结构聚1- NiO x材料的物理,化学和电催化性能。将结果与通过相同的电化学程序直接沉积在裸电极(无聚合物)上的NiO x的结果进行了比较。这样的表征清楚地证明了poly 1基质产生较小且无团聚的NiO x颗粒的有益作用。由于NiO x纳米粒子的尺寸较小(约21 nm)和在碳(C)电极表面沉积的聚1- NiO x复合膜的良好纳米结构,这种材料在接近中性的溶液(pH 9.2)甚至在碱性溶液中均表现出很高的电催化OER性能。 pH值14)具有很强的质量活度和转换频率(TOFs)(分别在pH 9.2和0.61 V的过电势下为1.12 A mg -1和0.17 s -1以及1.28 A mg -1和0.19 s -1,分别在pH 14的0.35 V过电势下)。此性能将C / poly 1 –NiO x文献中报道的基于氧化镍的活性最高的阳极中的电极,并且在OER中未掺杂铁离子。此外,当在多孔ITO电极上沉积多1 -NiO x膜时,如在1.2 V vs. Ag / AgCl的条件下进行长期电催化所显示的,其在电极表面的物理吸附显着增强。的确,经过数天的电催化后,其催化电流保持恒定,这表明了聚1- NiO x纳米复合材料的出色稳定性。聚合物基体保持NiO x的完整性纳米颗粒并防止其腐蚀。因此,这种材料对于实现有效且高度稳定的水氧化阳极非常有前途。











































 京公网安备 11010802027423号
京公网安备 11010802027423号