当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Porphyromonas gingivalis Gingipains Display Transpeptidation Activity
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-07-20 , DOI: 10.1021/acs.jproteome.8b00286 Lianyi Zhang 1 , Paul D. Veith 1 , N. Laila Huq 1 , Yu-Yen Chen 1 , Christine A. Seers 1 , Keith J. Cross 1 , Dhana G. Gorasia 1 , Eric C. Reynolds 1
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-07-20 , DOI: 10.1021/acs.jproteome.8b00286 Lianyi Zhang 1 , Paul D. Veith 1 , N. Laila Huq 1 , Yu-Yen Chen 1 , Christine A. Seers 1 , Keith J. Cross 1 , Dhana G. Gorasia 1 , Eric C. Reynolds 1
Affiliation
|
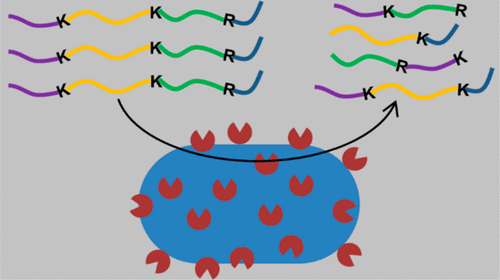
Porphyromonas gingivalis is a keystone periodontal pathogen that has been associated with autoimmune disorders. The cell surface proteases Lys-gingipain (Kgp) and Arg-gingipains (RgpA and RgpB) are major virulence factors, and their proteolytic activity is enhanced by small peptides such as glycylglycine (GlyGly). The reaction kinetics suggested that GlyGly may function as an acceptor molecule for gingipain-catalyzed transpeptidation. Purified gingipains and P. gingivalis whole cells were used to digest selected substrates including human hemoglobin in the presence or absence of peptide acceptors. Mass spectrometric analysis of the substrates digested with gingipains in the presence of GlyGly showed that transpeptidation outcompeted hydrolysis, whereas the trypsin-digested controls exhibited predominantly hydrolysis activity. The transpeptidation levels increased with increasing concentration of GlyGly. Purified gingipains and whole cells exhibited extensive transpeptidation activities on human hemoglobin. All hemoglobin cleavage sites were found to be suitable for GlyGly transpeptidation, and this transpeptidation enhanced hemoglobin digestion. The transpeptidation products were often more abundant than the corresponding hydrolysis products. In the absence of GlyGly, hemoglobin peptides produced during digestion were utilized as acceptors leading to the detection of up to 116 different transpeptidation products in a single reaction. P. gingivalis cells were able to digest hemoglobin faster when acceptor peptides derived from human serum albumin were included in the reaction, suggesting that gingipain-catalyzed transpeptidation may be relevant for substrates encountered in vivo. The transpeptidation of host proteins in vivo may potentially lead to the breakdown of immunological tolerance, culminating in autoimmune reactions.
中文翻译:

牙龈卟啉单胞菌姜黄素显示出转肽活性
牙龈卟啉单胞菌(Porphyromonas gingivalis)是与自身免疫性疾病相关的关键性牙周病原体。细胞表面蛋白酶Lys-gingipain(Kgp)和Arg-gingipains(RgpA和RgpB)是主要的毒力因子,它们的蛋白水解活性被诸如甘氨酰甘氨酸(GlyGly)之类的小肽增强。反应动力学表明,GlyGly可能充当姜黄素催化的转肽反应的受体分子。纯化的gingipains和P. gingivalis在存在或不存在肽受体的情况下,将全细胞用于消化选定的底物,包括人血红蛋白。在GlyGly存在下,用姜黄素消化的底物的质谱分析表明,转肽作用胜过水解,而胰蛋白酶消化的对照则主要表现出水解活性。转肽水平随GlyGly浓度的增加而增加。纯化的姜黄素和全细胞对人血红蛋白表现出广泛的转肽活性。发现所有的血红蛋白切割位点都适合于GlyGly转肽作用,并且这种转肽作用增强了血红蛋白消化。转肽产物通常比相应的水解产物丰富。在没有GlyGly的情况下,当反应中包含源自人血清白蛋白的受体肽时,牙龈卟啉单胞菌细胞能够更快地消化血红蛋白,表明牙龈蛋白酶催化的转肽作用可能与体内遇到的底物有关。体内宿主蛋白的转肽可能会导致免疫耐受性下降,最终导致自身免疫反应。
更新日期:2018-07-21
中文翻译:

牙龈卟啉单胞菌姜黄素显示出转肽活性
牙龈卟啉单胞菌(Porphyromonas gingivalis)是与自身免疫性疾病相关的关键性牙周病原体。细胞表面蛋白酶Lys-gingipain(Kgp)和Arg-gingipains(RgpA和RgpB)是主要的毒力因子,它们的蛋白水解活性被诸如甘氨酰甘氨酸(GlyGly)之类的小肽增强。反应动力学表明,GlyGly可能充当姜黄素催化的转肽反应的受体分子。纯化的gingipains和P. gingivalis在存在或不存在肽受体的情况下,将全细胞用于消化选定的底物,包括人血红蛋白。在GlyGly存在下,用姜黄素消化的底物的质谱分析表明,转肽作用胜过水解,而胰蛋白酶消化的对照则主要表现出水解活性。转肽水平随GlyGly浓度的增加而增加。纯化的姜黄素和全细胞对人血红蛋白表现出广泛的转肽活性。发现所有的血红蛋白切割位点都适合于GlyGly转肽作用,并且这种转肽作用增强了血红蛋白消化。转肽产物通常比相应的水解产物丰富。在没有GlyGly的情况下,当反应中包含源自人血清白蛋白的受体肽时,牙龈卟啉单胞菌细胞能够更快地消化血红蛋白,表明牙龈蛋白酶催化的转肽作用可能与体内遇到的底物有关。体内宿主蛋白的转肽可能会导致免疫耐受性下降,最终导致自身免疫反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号