当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogenation of Furfural with Nickel Nanoparticles Stabilized on Nitrogen-Rich Carbon Core–Shell and Its Transformations for the Synthesis of γ-Valerolactone in Aqueous Conditions
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1021/acsami.8b04239 Sekhar Nandi , Arka Saha , Parth Patel 1 , Noor-ul H. Khan 1 , Rukhsana I. Kureshy , Asit B. Panda
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1021/acsami.8b04239 Sekhar Nandi , Arka Saha , Parth Patel 1 , Noor-ul H. Khan 1 , Rukhsana I. Kureshy , Asit B. Panda
Affiliation
|
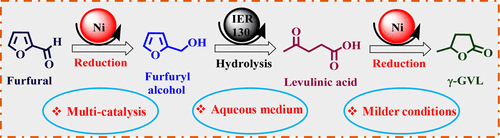
In this article, we report the synthesis of nitrogen-rich carbon layer-encapsulated Ni(0) nanoparticles as a core–shell structure ([email protected]/C-g-800) for the catalytic hydrogenation of furfural to furfuryl alcohol. The nickel nanoparticles were stabilized by the nitrogen-rich graphitic framework, which formed during the agitation of nickel acetate-impregnated cucurbit[6]uril surface in a reducing atmosphere. Furthermore, the catalyst was characterized using various physicochemical methods such as powder X-ray diffraction, Raman, field emission-scanning electron microscopy, high-resolution transmission electron microscopy, X-ray photoelectron spectroscopy, Brunauer–Emmett–Teller surface area, CO2-temperature-programmed desorption, inductive coupled plasma, and CHN analyses. The nitrogen-rich environment of the solid support with metallic Ni nanoparticles was found to be active and selective for the catalytic hydrogenation of furfural with molecular H2 in an aqueous medium at 100 °C. To understand the reaction mechanism, the diffuse reflectance infrared Fourier transform study was performed, which revealed that the C═O bond is activated in the presence of a catalyst. In addition, we have extended our methodology toward the synthesis of “levulinic acid” and “γ-valerolactone”, by successive hydrolysis and hydrogenation of furfuryl alcohol and levulinic acid, respectively, in an aqueous medium. Moreover, the heterogeneous catalysts used in all of the three consecutive steps help in recovery and recycling of the catalyst and easy separation of products.
中文翻译:

富氮碳核-壳稳定的镍纳米粒子氢化糠醛及其在水中合成γ-戊内酯的转化
在本文中,我们报告了富氮碳层包封的Ni(0)纳米颗粒的合成,该纳米颗粒作为核壳结构([email protected] / Cg-800)用于糠醛催化加氢成糠醇。镍纳米粒子通过富氮石墨骨架而稳定,该骨架是在还原性气氛中搅拌醋酸镍浸渍的葫芦丝[6]尿素表面时形成的。此外,使用各种物理化学方法对催化剂进行了表征,例如粉末X射线衍射,拉曼光谱,场发射扫描电子显微镜,高分辨率透射电子显微镜,X射线光电子能谱,Brunauer–Emmett–Teller表面积,CO 2程序升温解吸,电感耦合等离子体和CHN分析。发现具有金属Ni纳米颗粒的固体载体的富氮环境对于用分子H 2进行糠醛的催化加氢具有活性和选择性。在100°C的水性介质中。为了理解反应机理,进行了漫反射红外傅里叶变换研究,结果表明C═O键在催化剂存在下被活化。另外,我们已经通过在水性介质中分别连续水解和氢化糠醇和乙酰丙酸,将方法扩展到合成“乙酰丙酸”和“γ-戊内酯”。此外,在所有三个连续步骤中使用的非均相催化剂有助于催化剂的回收和再循环以及产物的容易分离。
更新日期:2018-07-06
中文翻译:

富氮碳核-壳稳定的镍纳米粒子氢化糠醛及其在水中合成γ-戊内酯的转化
在本文中,我们报告了富氮碳层包封的Ni(0)纳米颗粒的合成,该纳米颗粒作为核壳结构([email protected] / Cg-800)用于糠醛催化加氢成糠醇。镍纳米粒子通过富氮石墨骨架而稳定,该骨架是在还原性气氛中搅拌醋酸镍浸渍的葫芦丝[6]尿素表面时形成的。此外,使用各种物理化学方法对催化剂进行了表征,例如粉末X射线衍射,拉曼光谱,场发射扫描电子显微镜,高分辨率透射电子显微镜,X射线光电子能谱,Brunauer–Emmett–Teller表面积,CO 2程序升温解吸,电感耦合等离子体和CHN分析。发现具有金属Ni纳米颗粒的固体载体的富氮环境对于用分子H 2进行糠醛的催化加氢具有活性和选择性。在100°C的水性介质中。为了理解反应机理,进行了漫反射红外傅里叶变换研究,结果表明C═O键在催化剂存在下被活化。另外,我们已经通过在水性介质中分别连续水解和氢化糠醇和乙酰丙酸,将方法扩展到合成“乙酰丙酸”和“γ-戊内酯”。此外,在所有三个连续步骤中使用的非均相催化剂有助于催化剂的回收和再循环以及产物的容易分离。











































 京公网安备 11010802027423号
京公网安备 11010802027423号