当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Glycal Metallanitrenes for 2-Amino Sugar Synthesis: Amidoglycosylation of Gulal-, Allal-, Glucal-, and Galactal 3-Carbamates
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1021/acs.joc.8b00893 Simran Buttar 1 , Julia Caine 1 , Evelyne Goné 1 , Reneé Harris 1 , Jennifer Gillman 1 , Roxanne Atienza 1 , Ritu Gupta 1 , Kimberly M Sogi 1 , Lauren Jain 1 , Nadia C Abascal 1 , Yetta Levine 1 , Lindsay M Repka 1 , Christian M Rojas 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1021/acs.joc.8b00893 Simran Buttar 1 , Julia Caine 1 , Evelyne Goné 1 , Reneé Harris 1 , Jennifer Gillman 1 , Roxanne Atienza 1 , Ritu Gupta 1 , Kimberly M Sogi 1 , Lauren Jain 1 , Nadia C Abascal 1 , Yetta Levine 1 , Lindsay M Repka 1 , Christian M Rojas 1
Affiliation
|
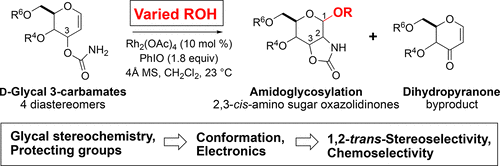
The rhodium(II)-catalyzed oxidative cyclization of glycal 3-carbamates with in situ incorporation of an alcohol nucleophile at the anomeric position provides access to a range of 2-amino sugars having 1,2-trans-2,3-cis stereochemistry, a structural motif present in compounds of medicinal and biological significance such as the streptothricin group of antibiotics and the Chitinase inhibitor allosamidin. All of the diastereomeric d-glycal 3-carbamates have been investigated, revealing significant differences in anomeric stereoselectivity depending on substrate stereochemistry and protecting groups. In addition, some substrates were prone to forming C3-oxidized dihydropyranone byproducts under the reaction conditions. Allal- and gulal 3-carbamates provided uniformly high stereo- and chemoselectivity, while for glucal substrates, acyclic, electron-withdrawing protecting groups at the 4O and 6O positions were required. Galactal 3-carbamates have been the most challenging substrates; formation of their amidoglycosylation products is most effective with an electron-withdrawing 6O-Ts substituent and a sterically demanding 4O-TBS group. These results suggest a mechanism whereby conformational and electronic factors determine the partitioning of an intermediate acyl nitrenoid between alkene addition, leading to amidoglycosylation, and C3–H insertion, providing the dihydropyranone byproduct. Along the amidoglycosylation pathway, high anomeric selectivity results when a glycosyl aziridine intermediate is favored over an aziridine-opened oxocarbenium donor.
中文翻译:

用于 2-氨基糖合成的糖基金属氮化合物:Gulal-、Allal-、Glucal- 和 Galactal 3-氨基甲酸酯的酰胺糖基化
铑 (II) 催化的糖基 3-氨基甲酸酯的氧化环化以及在异头位置原位掺入醇亲核试剂提供了一系列具有 1,2-反式-2,3-顺式立体化学的 2-氨基糖,存在于具有医学和生物学意义的化合物中的结构基序,例如抗生素链丝菌素组和几丁质酶抑制剂异糖脒。所有非对映异构体d-糖基 3-氨基甲酸酯均经过研究,揭示了端基异构立体选择性的显着差异,具体取决于底物立体化学和保护基团。此外,一些底物在反应条件下容易形成C3-氧化的二氢吡喃酮副产物。 Allal-和gulal 3-氨基甲酸酯提供了一致的高立体选择性和化学选择性,而对于葡萄糖底物,需要在4 O和6 O位置上的无环吸电子保护基团。半乳糖 3-氨基甲酸酯是最具挑战性的底物;其酰胺糖基化产物的形成对于吸电子 6 O -Ts 取代基和空间要求较高的 4 O -TBS 基团最为有效。这些结果表明构象和电子因素决定了中间体酰基氮烯类化合物在烯烃加成(导致酰胺基糖基化)和 C3-H 插入(提供二氢吡喃酮副产物)之间的分配。沿着酰胺糖基化途径,当糖基氮丙啶中间体优于氮丙啶开放的氧碳鎓供体时,会产生高异头选择性。
更新日期:2018-07-06
中文翻译:

用于 2-氨基糖合成的糖基金属氮化合物:Gulal-、Allal-、Glucal- 和 Galactal 3-氨基甲酸酯的酰胺糖基化
铑 (II) 催化的糖基 3-氨基甲酸酯的氧化环化以及在异头位置原位掺入醇亲核试剂提供了一系列具有 1,2-反式-2,3-顺式立体化学的 2-氨基糖,存在于具有医学和生物学意义的化合物中的结构基序,例如抗生素链丝菌素组和几丁质酶抑制剂异糖脒。所有非对映异构体d-糖基 3-氨基甲酸酯均经过研究,揭示了端基异构立体选择性的显着差异,具体取决于底物立体化学和保护基团。此外,一些底物在反应条件下容易形成C3-氧化的二氢吡喃酮副产物。 Allal-和gulal 3-氨基甲酸酯提供了一致的高立体选择性和化学选择性,而对于葡萄糖底物,需要在4 O和6 O位置上的无环吸电子保护基团。半乳糖 3-氨基甲酸酯是最具挑战性的底物;其酰胺糖基化产物的形成对于吸电子 6 O -Ts 取代基和空间要求较高的 4 O -TBS 基团最为有效。这些结果表明构象和电子因素决定了中间体酰基氮烯类化合物在烯烃加成(导致酰胺基糖基化)和 C3-H 插入(提供二氢吡喃酮副产物)之间的分配。沿着酰胺糖基化途径,当糖基氮丙啶中间体优于氮丙啶开放的氧碳鎓供体时,会产生高异头选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号