当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transformations of Isonitriles with Bromoalkanes Using Photoredox Gold Catalysis
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1021/acs.joc.8b01380 Samantha Rohe 1 , Terry McCallum 1 , Avery O. Morris 1 , Louis Barriault 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1021/acs.joc.8b01380 Samantha Rohe 1 , Terry McCallum 1 , Avery O. Morris 1 , Louis Barriault 1
Affiliation
|
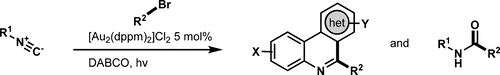
Isonitriles have excellent electronic compatibility to react with free radicals. Recently, photoredox catalysis has emerged as a powerful tool for the construction of C–C bonds with few protocols for alkylative heterocycle synthesis through isonitrile addition. Herein, we describe the photocatalytic generation of alkyl radicals from unactivated bromoalkanes as part of an efficient cross-coupling strategy for the diversification of isonitriles using a dimeric gold(I) photoredox catalyst, [Au2(dppm)2]Cl2.
中文翻译:

光氧化还原金催化溴代烷烃转化异腈
异腈具有出色的电子相容性,可与自由基反应。最近,光氧化还原催化已成为构建C-C键的有力工具,很少有通过异腈加成合成烷基化杂环的方案。在这里,我们描述了使用二聚金(I)光氧化还原催化剂[Au 2(dppm)2 ] Cl 2,从未活化的溴代烷烃中光催化生成烷基自由基的方法,这是一种有效的交叉交叉策略,可实现多种异腈的交叉偶联。
更新日期:2018-07-06
中文翻译:

光氧化还原金催化溴代烷烃转化异腈
异腈具有出色的电子相容性,可与自由基反应。最近,光氧化还原催化已成为构建C-C键的有力工具,很少有通过异腈加成合成烷基化杂环的方案。在这里,我们描述了使用二聚金(I)光氧化还原催化剂[Au 2(dppm)2 ] Cl 2,从未活化的溴代烷烃中光催化生成烷基自由基的方法,这是一种有效的交叉交叉策略,可实现多种异腈的交叉偶联。











































 京公网安备 11010802027423号
京公网安备 11010802027423号