当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkali-driven disassembly and reassembly of molecular niobium oxide in water
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-05 , DOI: 10.1021/jacs.8b05015 Dylan Sures 1 , Mireia Segado 2 , Carles Bo 2, 3 , May Nyman 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-05 , DOI: 10.1021/jacs.8b05015 Dylan Sures 1 , Mireia Segado 2 , Carles Bo 2, 3 , May Nyman 1
Affiliation
|
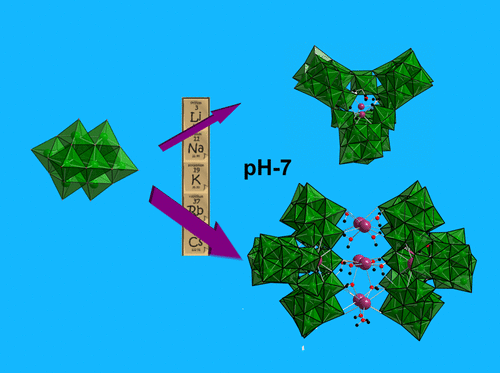
Counterions are deemed "spectators" in aqueous solutions of cationic or anionic molecular metal-oxo clusters. While pH and concentration drive aqueous metal speciation as a first approximation, the important effect of counterions is usually overlooked and never considered in standard Pourbaix databases. Alkali counterions for polyoxometalate (POM) clusters control solubility with distinct periodic trends, but evidence for alkali control over speciation is ambiguous. Here we show that a simple Nb-POM, [Nb10O28]6- ({Nb10}), converts to oligomers of (H xNb24O72)(24- x)- ({Nb24}) upon adding only alkali chloride salts, even in buffered neutral solutions. Raman and X-ray scattering reveal that the rate of {Nb10} to {Nb24} conversion increases with alkali cation radius and cation concentration. Cation-bridged oligomers of {Nb24} y ( y = 2,4) are defined by comparing experimental to computed small-angle X-ray scattering spectra. Computational studies and mass spectrometry indicate that the alkalis open the compact {Nb10} cluster in conjunction with protonation of a heptamer {Nb7} intermediate, in which alkali-{Nb10} association at key locations on the cluster initiates the reaction. Computation also explains the alkali periodic trend for {Nb10} to {Nb24} conversion; larger alkalis more effectively destabilize {Nb10}. This periodic trend asserts the hypothesis that Nb-cluster speciation near neutral pH is driven by the alkali cations in the absence of added base or acid. The extremely high solubility of these 3.5 nm polyoxoanion assemblies-2 M Nb at near neutral pH-is both surprising and exploitable for aqueous synthesis of niobate thin films or nanomaterials used in energy and microelectronics applications.
中文翻译:

水中氧化铌分子的碱驱动分解和重组
抗衡离子被认为是阳离子或阴离子分子金属氧簇的水溶液中的“旁观者”。虽然 pH 值和浓度驱动水性金属形态作为第一个近似值,但抗衡离子的重要影响通常被忽视,并且在标准的 Pourbaix 数据库中从未考虑过。多金属氧酸盐 (POM) 簇的碱反离子以明显的周期性趋势控制溶解度,但碱控制物种形成的证据不明确。在这里,我们展示了一个简单的 Nb-POM,[Nb10O28]6- ({Nb10}),在仅添加碱金属氯化物盐时,即使在缓冲液中也能转化为 (H xNb24O72)(24- x)- ({Nb24}) 的低聚物中性解决方案。拉曼和 X 射线散射表明 {Nb10} 到 {Nb24} 的转化率随着碱金属阳离子半径和阳离子浓度的增加而增加。{Nb24} y ( y = 2, 4) 通过将实验与计算的小角度 X 射线散射光谱进行比较来定义。计算研究和质谱表明,碱与七聚体 {Nb7} 中间体的质子化一起打开紧密的 {Nb10} 簇,其中簇上关键位置的碱-{Nb10} 缔合引发反应。计算还解释了 {Nb10} 到 {Nb24} 转化的碱周期趋势;较大的碱更有效地破坏 {Nb10}。这种周期性趋势表明,在不添加碱或酸的情况下,接近中性 pH 值的 Nb 簇物种形成是由碱性阳离子驱动的。这3种溶解度极高。
更新日期:2018-07-05
中文翻译:

水中氧化铌分子的碱驱动分解和重组
抗衡离子被认为是阳离子或阴离子分子金属氧簇的水溶液中的“旁观者”。虽然 pH 值和浓度驱动水性金属形态作为第一个近似值,但抗衡离子的重要影响通常被忽视,并且在标准的 Pourbaix 数据库中从未考虑过。多金属氧酸盐 (POM) 簇的碱反离子以明显的周期性趋势控制溶解度,但碱控制物种形成的证据不明确。在这里,我们展示了一个简单的 Nb-POM,[Nb10O28]6- ({Nb10}),在仅添加碱金属氯化物盐时,即使在缓冲液中也能转化为 (H xNb24O72)(24- x)- ({Nb24}) 的低聚物中性解决方案。拉曼和 X 射线散射表明 {Nb10} 到 {Nb24} 的转化率随着碱金属阳离子半径和阳离子浓度的增加而增加。{Nb24} y ( y = 2, 4) 通过将实验与计算的小角度 X 射线散射光谱进行比较来定义。计算研究和质谱表明,碱与七聚体 {Nb7} 中间体的质子化一起打开紧密的 {Nb10} 簇,其中簇上关键位置的碱-{Nb10} 缔合引发反应。计算还解释了 {Nb10} 到 {Nb24} 转化的碱周期趋势;较大的碱更有效地破坏 {Nb10}。这种周期性趋势表明,在不添加碱或酸的情况下,接近中性 pH 值的 Nb 簇物种形成是由碱性阳离子驱动的。这3种溶解度极高。









































 京公网安备 11010802027423号
京公网安备 11010802027423号