当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Conjugation of Amphiphilic Proteins to Hydrophobic Ligands in Organic Solvent
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00354 Antonietta M. Lillo,Ciana L. Lopez,Trideep Rajale,Hung-Ju Yen,Harsha D. Magurudeniya,M. Lisa Phipps,Eva Rose M. Balog,Timothy C. Sanchez,Srinivas Iyer,Hsing-Lin Wang,Ryszard Michalczyk,Reginaldo C. Rocha,Jennifer S. Martinez
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00354 Antonietta M. Lillo,Ciana L. Lopez,Trideep Rajale,Hung-Ju Yen,Harsha D. Magurudeniya,M. Lisa Phipps,Eva Rose M. Balog,Timothy C. Sanchez,Srinivas Iyer,Hsing-Lin Wang,Ryszard Michalczyk,Reginaldo C. Rocha,Jennifer S. Martinez
|
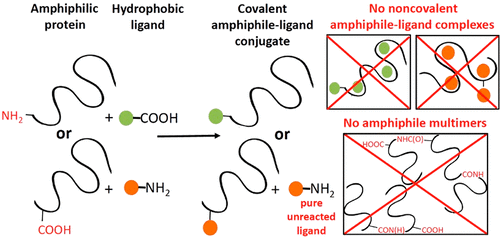
Protein–ligand conjugations are usually carried out in aqueous media in order to mimic the environment within which the conjugates will be used. In this work, we focus on the conjugation of amphiphilic variants of elastin-like polypeptide (ELP), short elastin (sEL), to poorly water-soluble compounds like OPPVs (p-phenylenevinylene oligomers), triarylamines, and polypyridine-metal complexes. These conjugations are problematic when carried out in aqueous phase because hydrophobic ligands tend to avoid exposure to water, which in turn causes the ligand to self-aggregate and/or interact noncovalently with hydrophobic regions of the amphiphile. Ultimately, this behavior leads to low conjugation efficiency and contamination with strong noncovalent “conjugates”. After exploring the solubility of sEL in various organic solvents, we have established an efficient conjugation methodology for obtaining covalent conjugates virtually free of contaminating noncovalent complexes. When conjugating carboxylated ligands to the amphiphile amines, we demonstrate that even when only one amine (the N-terminus) is present, its derivatization is 98% efficient. When conjugating amine moieties to the amphiphile carboxyls (a problematic configuration), protein multimerization is avoided, 98–100% of the protein is conjugated, and the unreacted ligand is recovered in pure form. Our syntheses occur in “one pot”, and our purification procedure is a simple workup utilizing a combination of water and organic solvent extractions. This conjugation methodology might provide a solution to problems arising from solubility mismatch of protein and ligand, and it is likely to be widely applied for modification of recombinant amphiphiles used for drug delivery (PEG-antibodies, polymer-enzymes, food proteins), cell adhesion (collagen, hydrophobins), synthesis of nanostructures (peptides), and engineering of biocompatible optoelectronics (biological polymers), to cite a few.
中文翻译:

两亲蛋白在有机溶剂中与疏水配体的结合
蛋白质-配体的偶联通常在水性介质中进行,以模拟偶联物的使用环境。在这项工作中,我们专注于弹性蛋白样多肽(ELP),短弹性蛋白(sEL)的两亲变体与水溶性差的化合物如OPPVs(p-亚苯基亚乙烯基低聚物),三芳基胺和聚吡啶-金属络合物。当在水相中进行时,这些缀合是有问题的,因为疏水性配体倾向于避免暴露于水,这继而引起配体自聚集和/或与两亲物的疏水性区域非共价相互作用。最终,这种行为导致结合效率低下,并被强力的非共价“结合物”污染。探索了sEL在各种有机溶剂中的溶解度后,我们建立了一种有效的偶联方法,可用于获得实际上不含污染性非共价复合物的共价结合物。当将羧基化的配体与两亲性胺缀合时,我们证明即使仅存在一种胺(N末端),其衍生化效率也达到98%。当将胺部分与两亲性羧基缀合时(有问题的构型),可以避免蛋白质多聚化,将98-100%的蛋白质缀合,并以纯净的形式回收未反应的配体。我们的合成过程在“一锅”中进行,我们的纯化程序是将水和有机溶剂提取物结合使用的简单后处理方法。这种缀合方法可以为蛋白质和配体的溶解度不匹配所引起的问题提供解决方案,并且可能广泛应用于修饰用于药物递送的重组两亲物(PEG抗体,聚合物酶,食物蛋白),细胞粘附(胶原蛋白,疏水蛋白),纳米结构(肽)的合成以及生物相容性光电器件(生物聚合物)的工程,仅举几例。
更新日期:2018-07-06
中文翻译:

两亲蛋白在有机溶剂中与疏水配体的结合
蛋白质-配体的偶联通常在水性介质中进行,以模拟偶联物的使用环境。在这项工作中,我们专注于弹性蛋白样多肽(ELP),短弹性蛋白(sEL)的两亲变体与水溶性差的化合物如OPPVs(p-亚苯基亚乙烯基低聚物),三芳基胺和聚吡啶-金属络合物。当在水相中进行时,这些缀合是有问题的,因为疏水性配体倾向于避免暴露于水,这继而引起配体自聚集和/或与两亲物的疏水性区域非共价相互作用。最终,这种行为导致结合效率低下,并被强力的非共价“结合物”污染。探索了sEL在各种有机溶剂中的溶解度后,我们建立了一种有效的偶联方法,可用于获得实际上不含污染性非共价复合物的共价结合物。当将羧基化的配体与两亲性胺缀合时,我们证明即使仅存在一种胺(N末端),其衍生化效率也达到98%。当将胺部分与两亲性羧基缀合时(有问题的构型),可以避免蛋白质多聚化,将98-100%的蛋白质缀合,并以纯净的形式回收未反应的配体。我们的合成过程在“一锅”中进行,我们的纯化程序是将水和有机溶剂提取物结合使用的简单后处理方法。这种缀合方法可以为蛋白质和配体的溶解度不匹配所引起的问题提供解决方案,并且可能广泛应用于修饰用于药物递送的重组两亲物(PEG抗体,聚合物酶,食物蛋白),细胞粘附(胶原蛋白,疏水蛋白),纳米结构(肽)的合成以及生物相容性光电器件(生物聚合物)的工程,仅举几例。



























 京公网安备 11010802027423号
京公网安备 11010802027423号