当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights on the Hydration of Terminal and Internal Allenes Catalyzed by [(NHC)Au]+
Organometallics ( IF 2.5 ) Pub Date : 2018-07-05 , DOI: 10.1021/acs.organomet.8b00230 Sara Muñoz-López 1 , Almudena Couce-Rios 1 , Giuseppe Sciortino 1, 2 , Agustí Lledós 1 , Gregori Ujaque 1
Organometallics ( IF 2.5 ) Pub Date : 2018-07-05 , DOI: 10.1021/acs.organomet.8b00230 Sara Muñoz-López 1 , Almudena Couce-Rios 1 , Giuseppe Sciortino 1, 2 , Agustí Lledós 1 , Gregori Ujaque 1
Affiliation
|
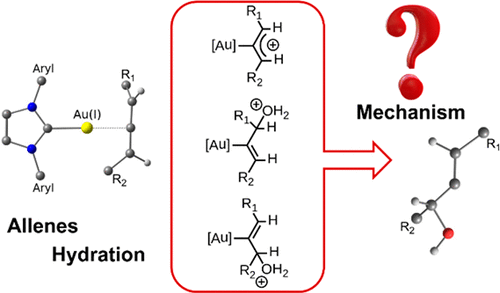
The reaction mechanism for the hydration of internal and terminal allenes catalyzed by [Au(NHC)]+ is analyzed by means of DFT calculations. Several reaction pathways for generating the two possible regioisomers were evaluated. Direct addition on coordinated allenes or to an intermediate with a σ-allylic cation structure as suggested for the Au(I)-catalyzed hydroamination of allenes were considered. The isomerization between both regioisomeric products catalyzed by the same Au(I) catalyst was also investigated as suggested for hydroalkoxylation of allenes. The regioselectivity of the reaction predicted by computation agrees with experiment for both terminal and internal allenes. The presence of alkyl or aryl substituents introduces differences in the reaction mechanism for the hydration process.
中文翻译:

[(NHC)Au] +催化末端和内部异戊烯水合的机理研究
通过DFT计算,分析了[Au(NHC)] +催化的内,外烯丙基水合反应的反应机理。评价了产生两种可能的区域异构体的几种反应途径。有人考虑将其直接加成到配位的烯基上,或直接添加到具有σ-烯丙基阳离子结构的中间体上,这是Au(I)催化的烯基加氢胺化所建议的。如对丙二烯的加氢烷氧基化所建议的,还研究了由相同的Au(I)催化剂催化的两种区域异构产物之间的异构化。通过计算预测的反应的区域选择性与末端和内部异位烯的实验一致。烷基或芳基取代基的存在在水合过程的反应机理上引入了差异。
更新日期:2018-07-08
中文翻译:

[(NHC)Au] +催化末端和内部异戊烯水合的机理研究
通过DFT计算,分析了[Au(NHC)] +催化的内,外烯丙基水合反应的反应机理。评价了产生两种可能的区域异构体的几种反应途径。有人考虑将其直接加成到配位的烯基上,或直接添加到具有σ-烯丙基阳离子结构的中间体上,这是Au(I)催化的烯基加氢胺化所建议的。如对丙二烯的加氢烷氧基化所建议的,还研究了由相同的Au(I)催化剂催化的两种区域异构产物之间的异构化。通过计算预测的反应的区域选择性与末端和内部异位烯的实验一致。烷基或芳基取代基的存在在水合过程的反应机理上引入了差异。










































 京公网安备 11010802027423号
京公网安备 11010802027423号