当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Electrochemical Surface Potential and Irradiation on Garnet-Type Almandine’s Dissolution Kinetics
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-07-19 , DOI: 10.1021/acs.jpcc.8b04459 Yi-Hsuan Hsiao , Erika Callagon La Plante , N. M. Anoop Krishnan 1 , Howard A. Dobbs , Yann Le Pape 2 , Narayanan Neithalath 3 , Mathieu Bauchy , Jacob Israelachvili , Gaurav Sant
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-07-19 , DOI: 10.1021/acs.jpcc.8b04459 Yi-Hsuan Hsiao , Erika Callagon La Plante , N. M. Anoop Krishnan 1 , Howard A. Dobbs , Yann Le Pape 2 , Narayanan Neithalath 3 , Mathieu Bauchy , Jacob Israelachvili , Gaurav Sant
Affiliation
|
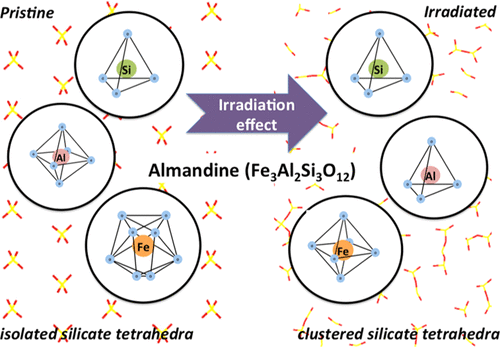
Nanoscale-resolved quantifications of almandine’s (Fe3Al2(SiO4)3) dissolution rates across a range of pHs (1 ≤ pH ≤ 13)—established using vertical scanning interferometry—reveal that its dissolution rate achieves a minimum around pH 5. This minimum coincides with almandine’s point of zero charge. These trends in almandine’s dissolution can be estimated using the Butler–Volmer equation that reveals linkages between surface potentials and dissolution rates, demonstrating proton- and hydroxyl-promoted breakage of Si–O bonds. In contrast to well-polymerized silicates, the dissolution of almandine can also occur through the rupture of its cationic bonds. This behavior is reflected in the observed influences of irradiation on its dissolution kinetics. Molecular dynamics simulations highlight that irradiation induces alterations in the atomic structure of almandine by reducing the coordination state of the cations (Fe2+ and Al3+), thereby enhancing its reactivity by a factor of two. This is consistent with the minor change induced in the structure of almandine’s silicate backbone, whose surface charge densities produce the observed pH dependence (and rate control) of dissolution rates. These findings reveal the influential roles of surface potential arising from solution pH and atomic scale alterations on affecting the reactivity of garnet-type silicates.
中文翻译:

电化学表面电势和辐照对石榴石型金刚烷溶解动力学的影响
Almandine's(Fe 3 Al 2(SiO 4)3)的纳米级定量分析)通过垂直扫描干涉法确定的在一定pH值(1≤pH≤13)范围内的溶出速率表明,其溶出速率在pH约为5时达到最小值。该最小值与艾曼丹碱的零电荷点相吻合。可以使用巴特勒-沃尔默方程式估算铝金刚烷溶解的这些趋势,该方程揭示了表面电势与溶解速率之间的联系,表明了质子和羟基促进的Si-O键断裂。与高度聚合的硅酸盐相反,金刚烷胺的溶解也可能通过其阳离子键的断裂而发生。这种行为反映在观察到的辐射对其溶解动力学的影响上。分子动力学模拟强调了辐射通过降低阳离子的配位态(Fe2+和Al 3+),从而将其反应性提高了两倍。这与艾曼丹碱的硅酸盐骨架的结构中引起的微小变化是一致的,其表面电荷密度产生了所观察到的溶解速率的pH依赖性(和速率控制)。这些发现揭示了溶液pH值和原子尺度变化引起的表面电势对石榴石型硅酸盐反应性的影响。
更新日期:2018-07-20
中文翻译:

电化学表面电势和辐照对石榴石型金刚烷溶解动力学的影响
Almandine's(Fe 3 Al 2(SiO 4)3)的纳米级定量分析)通过垂直扫描干涉法确定的在一定pH值(1≤pH≤13)范围内的溶出速率表明,其溶出速率在pH约为5时达到最小值。该最小值与艾曼丹碱的零电荷点相吻合。可以使用巴特勒-沃尔默方程式估算铝金刚烷溶解的这些趋势,该方程揭示了表面电势与溶解速率之间的联系,表明了质子和羟基促进的Si-O键断裂。与高度聚合的硅酸盐相反,金刚烷胺的溶解也可能通过其阳离子键的断裂而发生。这种行为反映在观察到的辐射对其溶解动力学的影响上。分子动力学模拟强调了辐射通过降低阳离子的配位态(Fe2+和Al 3+),从而将其反应性提高了两倍。这与艾曼丹碱的硅酸盐骨架的结构中引起的微小变化是一致的,其表面电荷密度产生了所观察到的溶解速率的pH依赖性(和速率控制)。这些发现揭示了溶液pH值和原子尺度变化引起的表面电势对石榴石型硅酸盐反应性的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号