当前位置:
X-MOL 学术
›
Mater. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Introduction of newly synthesized Schiff base molecules as efficient corrosion inhibitors for mild steel in 1 M HCl medium: an experimental, density functional theory and molecular dynamics simulation study†
Materials Chemistry Frontiers ( IF 6.0 ) Pub Date : 2018-07-05 00:00:00 , DOI: 10.1039/c8qm00162f Sourav Kr. Saha 1, 2, 3, 4, 5 , Priyabrata Banerjee 1, 2, 3, 4, 5
Materials Chemistry Frontiers ( IF 6.0 ) Pub Date : 2018-07-05 00:00:00 , DOI: 10.1039/c8qm00162f Sourav Kr. Saha 1, 2, 3, 4, 5 , Priyabrata Banerjee 1, 2, 3, 4, 5
Affiliation
|
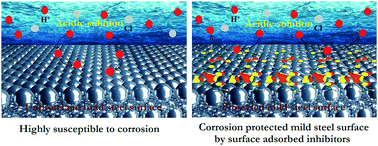
The purposeful incorporation of aliphatic, branched chain and substituted aromatic moieties in the molecular skeleton of organic Schiff bases, in line with corrosion inhibition performance, has been conducted. Three Schiff bases, namely, 2-((2-hydroxyethylimino)methyl)-6-methoxyphenol (L1), 2-((1-hydroxybutan-2-ylimino)methyl)-6-methoxyphenol (L2) and 2-(2-hydroxy-3-methoxybenzylideneamino)phenol (L3) were synthesized and subsequently assessed for the inhibition of mild steel corrosion in 1 M HCl medium. The corrosion inhibition proficiencies of the synthesized inhibitors on the mild steel surface have been investigated by gravimetric measurements, electrochemical analysis (potentiodynamic polarization, electrochemical impedance spectroscopy), surface morphological studies (FESEM and AFM), contact angle measurement and, most importantly, theoretical studies. The results obtained from the gravimetric as well as electrochemical measurements revealed that the studied Schiff bases are mixed-type inhibitors that show maximum inhibition efficiency up to ∼97% at the concentration of 5 mM. In theoretical studies, atomic-level calculations give deeper insights into the corrosion inhibition mechanism and the relative performance of present inhibitors. However, it has been found that normal density functional theory (DFT) calculations are not sufficient to deal with the interactions between inhibitors and metal surfaces in complex systems. As such, in order to investigate inhibitor–metal interactions, herein, the density functional tight binding (DFTB) approach has been introduced as formulated by Hohenberg, Kohn and Sham (KS-DFT). Furthermore, for more insightful studies, e.g., growth characteristics, as well as the selection of a more stable surface of the α-Fe crystal morphology studies, have also been performed using the “morphology” module. The morphology of the α-Fe crystal in equilibrium was analyzed using the Wulff construction plot. In DFTB calculations, the trans3d Slater–Koster library set has been implemented for possible pairs of interactions between inhibitor–metal complex systems (C, N, O, H and Fe). Some salient features like equilibrium adsorption configuration and charge density difference obtained from DFTB calculations have been described in detail. Furthermore, for comparison with the real world of corrosion inhibition, molecular dynamics (MD) simulation was employed in the presence of all concerned species (inhibitor molecule, Fe surface, H2O, H3O+ and Cl−), which revealed the actual adsorption configurations of the inhibitor molecule on the desired metallic surfaces.
中文翻译:

引入新合成的席夫碱分子作为1 M HCl介质中低碳钢的有效缓蚀剂:实验,密度泛函理论和分子动力学模拟研究†
根据腐蚀抑制性能,已经有目的地将脂族,支链和取代的芳族部分掺入有机席夫碱的分子骨架中。三个席夫碱,即2-(((2-羟乙基亚氨基)甲基)-6-甲氧基苯酚(L 1),2-(((1-羟丁基-2-ylimino)甲基)-6-甲氧基苯酚(L 2)和2- (2-羟基-3-甲氧基亚苄基氨基)苯酚(L 3合成),然后评估其在1 M HCl介质中对低碳钢腐蚀的抑制作用。通过重量分析,电化学分析(电位动力学极化,电化学阻抗谱),表面形态研究(FESEM和AFM),接触角测量以及最重要的理论研究,研究了合成缓蚀剂在低碳钢表面上的缓蚀性能。 。从重量法和电化学测量获得的结果表明,所研究的席夫碱是混合型抑制剂,在浓度为5 mM时显示最大抑制效率,最高可达约97%。在理论研究中,原子水平的计算可以更深入地了解缓蚀机理和目前抑制剂的相对性能。但是,已经发现,正常密度泛函理论(DFT)计算不足以处理复杂系统中抑制剂与金属表面之间的相互作用。因此,为了研究抑制剂与金属的相互作用,本文采用了Hohenberg,Kohn和Sham(KS-DFT)提出的密度泛函紧密结合(DFTB)方法。此外,对于更深入的研究,例如,还使用“形态”模块进行了生长特性以及对α-Fe晶体形态研究的更稳定表面的选择。使用Wulff构造图分析了处于平衡状态的α-Fe晶体的形貌。在DFTB计算中,针对抑制剂-金属复杂系统(C,N,O,H和Fe)之间可能存在的相互作用对,使用了trans3d Slater-Koster库集。已经详细描述了一些显着特征,如平衡吸附构型和从DFTB计算获得的电荷密度差。此外,为了与腐蚀抑制的现实世界进行比较,在所有相关物种(抑制剂分子,Fe表面,H 2O,H 3 ö +和Cl - ),其揭示了所需的金属表面上的抑制剂分子的实际吸附的配置。
更新日期:2018-07-05
中文翻译:

引入新合成的席夫碱分子作为1 M HCl介质中低碳钢的有效缓蚀剂:实验,密度泛函理论和分子动力学模拟研究†
根据腐蚀抑制性能,已经有目的地将脂族,支链和取代的芳族部分掺入有机席夫碱的分子骨架中。三个席夫碱,即2-(((2-羟乙基亚氨基)甲基)-6-甲氧基苯酚(L 1),2-(((1-羟丁基-2-ylimino)甲基)-6-甲氧基苯酚(L 2)和2- (2-羟基-3-甲氧基亚苄基氨基)苯酚(L 3合成),然后评估其在1 M HCl介质中对低碳钢腐蚀的抑制作用。通过重量分析,电化学分析(电位动力学极化,电化学阻抗谱),表面形态研究(FESEM和AFM),接触角测量以及最重要的理论研究,研究了合成缓蚀剂在低碳钢表面上的缓蚀性能。 。从重量法和电化学测量获得的结果表明,所研究的席夫碱是混合型抑制剂,在浓度为5 mM时显示最大抑制效率,最高可达约97%。在理论研究中,原子水平的计算可以更深入地了解缓蚀机理和目前抑制剂的相对性能。但是,已经发现,正常密度泛函理论(DFT)计算不足以处理复杂系统中抑制剂与金属表面之间的相互作用。因此,为了研究抑制剂与金属的相互作用,本文采用了Hohenberg,Kohn和Sham(KS-DFT)提出的密度泛函紧密结合(DFTB)方法。此外,对于更深入的研究,例如,还使用“形态”模块进行了生长特性以及对α-Fe晶体形态研究的更稳定表面的选择。使用Wulff构造图分析了处于平衡状态的α-Fe晶体的形貌。在DFTB计算中,针对抑制剂-金属复杂系统(C,N,O,H和Fe)之间可能存在的相互作用对,使用了trans3d Slater-Koster库集。已经详细描述了一些显着特征,如平衡吸附构型和从DFTB计算获得的电荷密度差。此外,为了与腐蚀抑制的现实世界进行比较,在所有相关物种(抑制剂分子,Fe表面,H 2O,H 3 ö +和Cl - ),其揭示了所需的金属表面上的抑制剂分子的实际吸附的配置。











































 京公网安备 11010802027423号
京公网安备 11010802027423号