Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19).
The Lancet ( IF 168.9 ) Pub Date : 2018-07-06 , DOI: 10.1016/s0140-6736(18)31364-3 Dan H Barouch 1 , Frank L Tomaka 2 , Frank Wegmann 3 , Daniel J Stieh 3 , Galit Alter 4 , Merlin L Robb 5 , Nelson L Michael 6 , Lauren Peter 7 , Joseph P Nkolola 7 , Erica N Borducchi 7 , Abishek Chandrashekar 7 , David Jetton 7 , Kathryn E Stephenson 1 , Wenjun Li 8 , Bette Korber 9 , Georgia D Tomaras 10 , David C Montefiori 10 , Glenda Gray 11 , Nicole Frahm 12 , M Juliana McElrath 12 , Lindsey Baden 13 , Jennifer Johnson 13 , Julia Hutter 14 , Edith Swann 14 , Etienne Karita 15 , Hannah Kibuuka 16 , Juliet Mpendo 17 , Nigel Garrett 18 , Kathy Mngadi 18 , Kundai Chinyenze 19 , Frances Priddy 19 , Erica Lazarus 10 , Fatima Laher 10 , Sorachai Nitayapan 20 , Punnee Pitisuttithum 21 , Stephan Bart 22 , Thomas Campbell 23 , Robert Feldman 24 , Gregg Lucksinger 25 , Caroline Borremans 26 , Katleen Callewaert 26 , Raphaele Roten 26 , Jerald Sadoff 3 , Lorenz Scheppler 27 , Mo Weijtens 3 , Karin Feddes-de Boer 3 , Daniëlle van Manen 3 , Jessica Vreugdenhil 3 , Roland Zahn 3 , Ludo Lavreys 26 , Steven Nijs 26 , Jeroen Tolboom 3 , Jenny Hendriks 3 , Zelda Euler 3 , Maria G Pau 3 , Hanneke Schuitemaker 3
The Lancet ( IF 168.9 ) Pub Date : 2018-07-06 , DOI: 10.1016/s0140-6736(18)31364-3 Dan H Barouch 1 , Frank L Tomaka 2 , Frank Wegmann 3 , Daniel J Stieh 3 , Galit Alter 4 , Merlin L Robb 5 , Nelson L Michael 6 , Lauren Peter 7 , Joseph P Nkolola 7 , Erica N Borducchi 7 , Abishek Chandrashekar 7 , David Jetton 7 , Kathryn E Stephenson 1 , Wenjun Li 8 , Bette Korber 9 , Georgia D Tomaras 10 , David C Montefiori 10 , Glenda Gray 11 , Nicole Frahm 12 , M Juliana McElrath 12 , Lindsey Baden 13 , Jennifer Johnson 13 , Julia Hutter 14 , Edith Swann 14 , Etienne Karita 15 , Hannah Kibuuka 16 , Juliet Mpendo 17 , Nigel Garrett 18 , Kathy Mngadi 18 , Kundai Chinyenze 19 , Frances Priddy 19 , Erica Lazarus 10 , Fatima Laher 10 , Sorachai Nitayapan 20 , Punnee Pitisuttithum 21 , Stephan Bart 22 , Thomas Campbell 23 , Robert Feldman 24 , Gregg Lucksinger 25 , Caroline Borremans 26 , Katleen Callewaert 26 , Raphaele Roten 26 , Jerald Sadoff 3 , Lorenz Scheppler 27 , Mo Weijtens 3 , Karin Feddes-de Boer 3 , Daniëlle van Manen 3 , Jessica Vreugdenhil 3 , Roland Zahn 3 , Ludo Lavreys 26 , Steven Nijs 26 , Jeroen Tolboom 3 , Jenny Hendriks 3 , Zelda Euler 3 , Maria G Pau 3 , Hanneke Schuitemaker 3
Affiliation
|
BACKGROUND
More than 1·8 million new cases of HIV-1 infection were diagnosed worldwide in 2016. No licensed prophylactic HIV-1 vaccine exists. A major limitation to date has been the lack of direct comparability between clinical trials and preclinical studies. We aimed to evaluate mosaic adenovirus serotype 26 (Ad26)-based HIV-1 vaccine candidates in parallel studies in humans and rhesus monkeys to define the optimal vaccine regimen to advance into clinical efficacy trials.
METHODS
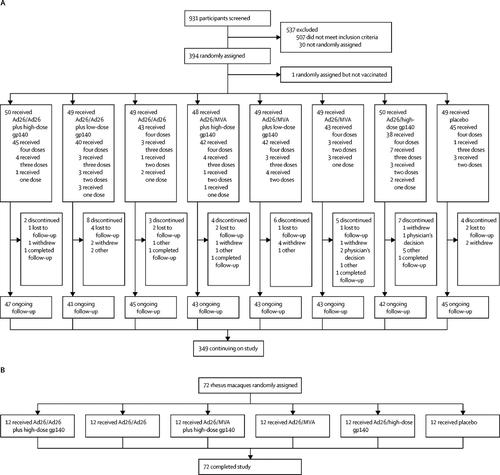
We conducted a multicentre, randomised, double-blind, placebo-controlled phase 1/2a trial (APPROACH). Participants were recruited from 12 clinics in east Africa, South Africa, Thailand, and the USA. We included healthy, HIV-1-uninfected participants (aged 18-50 years) who were considered at low risk for HIV-1 infection. We randomly assigned participants to one of eight study groups, stratified by region. Participants and investigators were blinded to the treatment allocation throughout the study. We primed participants at weeks 0 and 12 with Ad26.Mos.HIV (5 × 1010 viral particles per 0·5 mL) expressing mosaic HIV-1 envelope (Env)/Gag/Pol antigens and gave boosters at weeks 24 and 48 with Ad26.Mos.HIV or modified vaccinia Ankara (MVA; 108 plaque-forming units per 0·5 mL) vectors with or without high-dose (250 μg) or low-dose (50 μg) aluminium adjuvanted clade C Env gp140 protein. Those in the control group received 0·9% saline. All study interventions were administered intramuscularly. Primary endpoints were safety and tolerability of the vaccine regimens and Env-specific binding antibody responses at week 28. Safety and immunogenicity were also assessed at week 52. All participants who received at least one vaccine dose or placebo were included in the safety analysis; immunogenicity was analysed using the per-protocol population. We also did a parallel study in rhesus monkeys (NHP 13-19) to assess the immunogenicity and protective efficacy of these vaccine regimens against a series of six repetitive, heterologous, intrarectal challenges with a rhesus peripheral blood mononuclear cell-derived challenge stock of simian-human immunodeficiency virus (SHIV-SF162P3). The APPROACH trial is registered with ClinicalTrials.gov, number NCT02315703.
FINDINGS
Between Feb 24, 2015, and Oct 16, 2015, we randomly assigned 393 participants to receive at least one dose of study vaccine or placebo in the APPROACH trial. All vaccine regimens demonstrated favourable safety and tolerability. The most commonly reported solicited local adverse event was mild-to-moderate pain at the injection site (varying from 69% to 88% between the different active groups vs 49% in the placebo group). Five (1%) of 393 participants reported at least one grade 3 adverse event considered related to the vaccines: abdominal pain and diarrhoea (in the same participant), increased aspartate aminotransferase, postural dizziness, back pain, and malaise. The mosaic Ad26/Ad26 plus high-dose gp140 boost vaccine was the most immunogenic in humans; it elicited Env-specific binding antibody responses (100%) and antibody-dependent cellular phagocytosis responses (80%) at week 52, and T-cell responses at week 50 (83%). We also randomly assigned 72 rhesus monkeys to receive one of five different vaccine regimens or placebo in the NHP 13-19 study. Ad26/Ad26 plus gp140 boost induced similar magnitude, durability, and phenotype of immune responses in rhesus monkeys as compared with humans and afforded 67% protection against acquisition of SHIV-SF162P3 infection (two-sided Fisher's exact test p=0·007). Env-specific ELISA and enzyme-linked immunospot assay responses were the principal immune correlates of protection against SHIV challenge in monkeys.
INTERPRETATION
The mosaic Ad26/Ad26 plus gp140 HIV-1 vaccine induced comparable and robust immune responses in humans and rhesus monkeys, and it provided significant protection against repetitive heterologous SHIV challenges in rhesus monkeys. This vaccine concept is currently being evaluated in a phase 2b clinical efficacy study in sub-Saharan Africa (NCT03060629).
FUNDING
Janssen Vaccines & Prevention BV, National Institutes of Health, Ragon Institute of MGH, MIT and Harvard, Henry M Jackson Foundation for the Advancement of Military Medicine, US Department of Defense, and International AIDS Vaccine Initiative.
中文翻译:

在多中心,随机,双盲,安慰剂对照的1 / 2a期临床试验(APPROACH)和恒河猴(NHP 13-19)中评估镶嵌HIV-1疫苗。
背景技术在2016年,全球诊断出超过1·800万新的HIV-1感染病例。不存在许可的预防性HIV-1疫苗。迄今为止的主要限制是临床试验与临床前研究之间缺乏直接的可比性。我们的目的是在人类和恒河猴的平行研究中评估基于花叶腺病毒血清型26(Ad26)的HIV-1候选疫苗,以定义最佳疫苗方案以推进临床疗效试验。方法我们进行了一项多中心,随机,双盲,安慰剂对照的1 / 2a试验(APPROACH)。参与者是从东非,南非,泰国和美国的12家诊所招募的。我们纳入了健康的,未感染HIV-1的参与者(18至50岁),他们被认为感染HIV-1的风险较低。我们将参与者随机分配到按地区分层的八个研究组之一。在整个研究过程中,参与者和研究者对治疗分配视而不见。我们在第0和12周时用表达表达镶嵌HIV-1包膜(Env)/ Gag / Pol抗原的Ad26.Mos.HIV(每0·5 mL 5×1010病毒颗粒)灌注参与者,并在第24和48周时用Ad26加强免疫Mos.HIV或修饰的牛痘安卡拉(MVA;每0·5 mL含108个噬菌斑形成单位)载体,带有或不带有高剂量(250μg)或低剂量(50μg)铝佐剂C Env gp140蛋白。对照组接受0·9%生理盐水。所有研究干预均通过肌肉注射进行。主要终点是疫苗接种方案的安全性和耐受性以及第28周时的Env特异性结合抗体反应。在第52周时还评估了安全性和免疫原性。所有接受至少一种疫苗剂量或安慰剂的参与者都包括在安全性分析中。使用按方案人群分析免疫原性。我们还对恒河猴(NHP 13-19)进行了一项平行研究,以评估这些疫苗接种方案针对恒河猴外周血单核细胞衍生的猿猴的一系列六种重复性,异源性,直肠内挑战的免疫原性和保护效力。 -人免疫缺陷病毒(SHIV-SF162P3)。APPROACH试验已在ClinicalTrials.gov上注册,编号为NCT02315703。结果在2015年2月24日至2015年10月16日之间,我们在APPROACH试验中随机分配了393名参与者,以接受至少一剂研究疫苗或安慰剂。所有疫苗方案均显示出良好的安全性和耐受性。最常见的局部不良事件是注射部位的轻度至中度疼痛(不同活性组之间的疼痛从69%到88%,而安慰剂组为49%)。393名参与者中的五名(1%)报告了至少一种与疫苗相关的3级不良事件:腹痛和腹泻(同一名参与者),天冬氨酸转氨酶升高,体位头晕,背痛和不适。镶嵌Ad26 / Ad26加高剂量gp140加强疫苗是人类免疫原性最高的疫苗;它在第52周引起Env特异性结合抗体应答(100%)和抗体依赖性细胞吞噬反应(80%),在第50周引起T细胞应答(83%)。在NHP 13-19研究中,我们还随机分配了72只恒河猴来接受五种不同的疫苗方案或安慰剂之一。与人类相比,Ad26 / Ad26加gp140增强在恒河猴中诱导了相似的强度,持久性和免疫反应表型,并提供了67%的保护以防止获得SHIV-SF162P3感染(双面Fisher精确检验p = 0·007)。Env特异性ELISA和酶联免疫斑点测定反应是猴子抵抗SHIV攻击的主要免疫相关因子。解释Ad26 / Ad26加gp140 HIV-1嵌合马赛克疫苗在人和恒河猴中诱导了可比且强大的免疫反应,它为抵抗恒河猴中重复的异源SHIV攻击提供了重要的保护。目前,该疫苗概念正在撒哈拉以南非洲(NCT03060629)的2b期临床疗效研究中进行评估。资金Janssen疫苗与预防BV,美国国立卫生研究院,MGH,麻省理工学院和哈佛大学Ragon研究所,亨利·M杰克逊军事医学发展基金会,美国国防部和国际AIDS疫苗倡议。
更新日期:2018-07-20
中文翻译:

在多中心,随机,双盲,安慰剂对照的1 / 2a期临床试验(APPROACH)和恒河猴(NHP 13-19)中评估镶嵌HIV-1疫苗。
背景技术在2016年,全球诊断出超过1·800万新的HIV-1感染病例。不存在许可的预防性HIV-1疫苗。迄今为止的主要限制是临床试验与临床前研究之间缺乏直接的可比性。我们的目的是在人类和恒河猴的平行研究中评估基于花叶腺病毒血清型26(Ad26)的HIV-1候选疫苗,以定义最佳疫苗方案以推进临床疗效试验。方法我们进行了一项多中心,随机,双盲,安慰剂对照的1 / 2a试验(APPROACH)。参与者是从东非,南非,泰国和美国的12家诊所招募的。我们纳入了健康的,未感染HIV-1的参与者(18至50岁),他们被认为感染HIV-1的风险较低。我们将参与者随机分配到按地区分层的八个研究组之一。在整个研究过程中,参与者和研究者对治疗分配视而不见。我们在第0和12周时用表达表达镶嵌HIV-1包膜(Env)/ Gag / Pol抗原的Ad26.Mos.HIV(每0·5 mL 5×1010病毒颗粒)灌注参与者,并在第24和48周时用Ad26加强免疫Mos.HIV或修饰的牛痘安卡拉(MVA;每0·5 mL含108个噬菌斑形成单位)载体,带有或不带有高剂量(250μg)或低剂量(50μg)铝佐剂C Env gp140蛋白。对照组接受0·9%生理盐水。所有研究干预均通过肌肉注射进行。主要终点是疫苗接种方案的安全性和耐受性以及第28周时的Env特异性结合抗体反应。在第52周时还评估了安全性和免疫原性。所有接受至少一种疫苗剂量或安慰剂的参与者都包括在安全性分析中。使用按方案人群分析免疫原性。我们还对恒河猴(NHP 13-19)进行了一项平行研究,以评估这些疫苗接种方案针对恒河猴外周血单核细胞衍生的猿猴的一系列六种重复性,异源性,直肠内挑战的免疫原性和保护效力。 -人免疫缺陷病毒(SHIV-SF162P3)。APPROACH试验已在ClinicalTrials.gov上注册,编号为NCT02315703。结果在2015年2月24日至2015年10月16日之间,我们在APPROACH试验中随机分配了393名参与者,以接受至少一剂研究疫苗或安慰剂。所有疫苗方案均显示出良好的安全性和耐受性。最常见的局部不良事件是注射部位的轻度至中度疼痛(不同活性组之间的疼痛从69%到88%,而安慰剂组为49%)。393名参与者中的五名(1%)报告了至少一种与疫苗相关的3级不良事件:腹痛和腹泻(同一名参与者),天冬氨酸转氨酶升高,体位头晕,背痛和不适。镶嵌Ad26 / Ad26加高剂量gp140加强疫苗是人类免疫原性最高的疫苗;它在第52周引起Env特异性结合抗体应答(100%)和抗体依赖性细胞吞噬反应(80%),在第50周引起T细胞应答(83%)。在NHP 13-19研究中,我们还随机分配了72只恒河猴来接受五种不同的疫苗方案或安慰剂之一。与人类相比,Ad26 / Ad26加gp140增强在恒河猴中诱导了相似的强度,持久性和免疫反应表型,并提供了67%的保护以防止获得SHIV-SF162P3感染(双面Fisher精确检验p = 0·007)。Env特异性ELISA和酶联免疫斑点测定反应是猴子抵抗SHIV攻击的主要免疫相关因子。解释Ad26 / Ad26加gp140 HIV-1嵌合马赛克疫苗在人和恒河猴中诱导了可比且强大的免疫反应,它为抵抗恒河猴中重复的异源SHIV攻击提供了重要的保护。目前,该疫苗概念正在撒哈拉以南非洲(NCT03060629)的2b期临床疗效研究中进行评估。资金Janssen疫苗与预防BV,美国国立卫生研究院,MGH,麻省理工学院和哈佛大学Ragon研究所,亨利·M杰克逊军事医学发展基金会,美国国防部和国际AIDS疫苗倡议。



























 京公网安备 11010802027423号
京公网安备 11010802027423号