当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of 2-Chlorophenylacetic Acid in 12 Pure Solvents from T = (273.15/283.15 to 318.15) K: Determination and Modeling
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-07-06 , DOI: 10.1021/acs.jced.8b00139 Hua Huang 1, 2 , Haizhen Yang 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-07-06 , DOI: 10.1021/acs.jced.8b00139 Hua Huang 1, 2 , Haizhen Yang 1
Affiliation
|
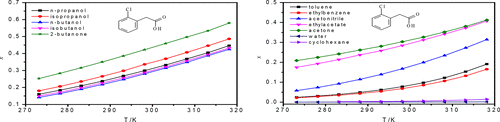
The solubility data of 2-chlorophenylacetic acid in solid–liquid equilibrium were indispensable for a preliminary industrial application aimed at purifying and renewing. In this paper, the solubilities of 2-chlorophenylacetic acid in 12 alternative solvents (toluene, water, acetonitrile, ethylacetate, ethylbenzene, n-propanol, isopropanol, n-butanol, isobutanol, cyclohexane, 2-butanone, and acetone) at atmospheric pressure and temperatures ranging from 273.15/283.15 to 318.15 K were determined by the isothermal saturation method. Upon the analysis of the experimental data, the mole fraction solubility increased when the solution temperature increased. Generally, the solubility order in different solvents is as follows: 2-butanone > (acetone, isopropanol, ethylacetate, n-propanol, isobutanol, n-butanol) > acetonitrile > toluene > ethylbenzene > cyclohexane > water. Thermodynamic models such as the modified Apelblat equation, λh equation, Wilson model, and NRTL model were chosen to fit and correlate the obtained solubility data. Some association parameter could be obtained. The maximum value of root-mean-square deviation (RMSD) is 48.72 × 10–4, and the maximum relative mean deviation (RAD) is 1.86%. Besides, the mixing properties of the solutions were obtained, including the mixing Gibbs energy, mixing enthalpy, mixing entropy, activity coefficient at infinitesimal concentration (γ1∞), and reduced excess enthalpy (H1E,∞). The course of dissolution is spontaneous, though the study of solubility and thermodynamics was helpful to the purification process of 2-chlorophenylacetic acid.
中文翻译:

T =(273.15 / 283.15至318.15)K中12种纯溶剂中的2-氯苯基乙酸的溶解度:确定和建模
2-氯苯基乙酸在固液平衡中的溶解度数据对于旨在纯化和更新的初步工业应用是必不可少的。本文研究了在常压下2-氯苯基乙酸在12种替代溶剂(甲苯,水,乙腈,乙酸乙酯,乙苯,正丙醇,异丙醇,正丁醇,异丁醇,环己烷,2-丁酮和丙酮)中的溶解度等温饱和法测定的温度范围为273.15 / 283.15至318.15K。根据实验数据分析,当溶液温度升高时,摩尔分数溶解度增加。通常,在不同溶剂中的溶解度顺序如下:2-丁酮>(丙酮,异丙醇,乙酸乙酯,正丁-丙醇,异丁醇,正丁醇)>乙腈>甲苯>乙苯>环己烷>水。热力学模型如改性Apelblat方程,λ ħ方程,Wilson模型,并且模型NRTL被选择,以适应和所获得的溶解度数据相关联。可以获得一些关联参数。均方根偏差(RMSD)的最大值为48.72×10 -4,最大相对平均偏差(RAD)为1.86%。此外,得到了溶液的混合性质,包括混合Gibbs能,混合焓,在无穷小的浓度混合熵,活度系数(γ 1 ∞),和减少过量的焓(ħ 1 E,∞)。溶解过程是自发的,尽管对溶解度和热力学的研究有助于2-氯苯基乙酸的纯化过程。
更新日期:2018-07-08
中文翻译:

T =(273.15 / 283.15至318.15)K中12种纯溶剂中的2-氯苯基乙酸的溶解度:确定和建模
2-氯苯基乙酸在固液平衡中的溶解度数据对于旨在纯化和更新的初步工业应用是必不可少的。本文研究了在常压下2-氯苯基乙酸在12种替代溶剂(甲苯,水,乙腈,乙酸乙酯,乙苯,正丙醇,异丙醇,正丁醇,异丁醇,环己烷,2-丁酮和丙酮)中的溶解度等温饱和法测定的温度范围为273.15 / 283.15至318.15K。根据实验数据分析,当溶液温度升高时,摩尔分数溶解度增加。通常,在不同溶剂中的溶解度顺序如下:2-丁酮>(丙酮,异丙醇,乙酸乙酯,正丁-丙醇,异丁醇,正丁醇)>乙腈>甲苯>乙苯>环己烷>水。热力学模型如改性Apelblat方程,λ ħ方程,Wilson模型,并且模型NRTL被选择,以适应和所获得的溶解度数据相关联。可以获得一些关联参数。均方根偏差(RMSD)的最大值为48.72×10 -4,最大相对平均偏差(RAD)为1.86%。此外,得到了溶液的混合性质,包括混合Gibbs能,混合焓,在无穷小的浓度混合熵,活度系数(γ 1 ∞),和减少过量的焓(ħ 1 E,∞)。溶解过程是自发的,尽管对溶解度和热力学的研究有助于2-氯苯基乙酸的纯化过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号