当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of the Temperature, Type of Salt, and Alcohol on Phase Diagrams of 2-Propanol + Inorganic Salt Aqueous Two-Phase Systems: Experimental Determination and Correlation
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-07-06 , DOI: 10.1021/acs.jced.8b00160 Seyyed Mohammad Arzideh 1 , Kamyar Movagharnejad 2 , Mohsen Pirdashti 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-07-06 , DOI: 10.1021/acs.jced.8b00160 Seyyed Mohammad Arzideh 1 , Kamyar Movagharnejad 2 , Mohsen Pirdashti 3
Affiliation
|
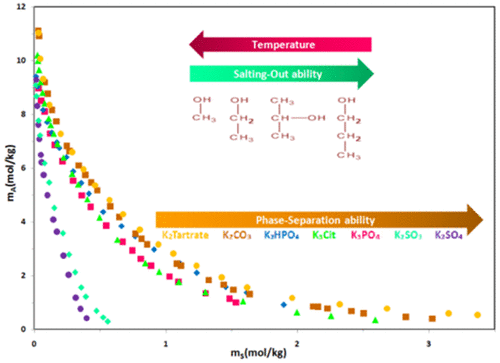
The liquid–liquid equilibriums (LLEs) for 2-propanol + tripotassium phosphate + water at 298.15, 308.15, and 318.15 K and 2-propanol + dipotassium hydrogen phosphate + water at 298.15 K were measured. The binodal curves of 2-propanol + potassium sulfate/potassium sulfite + water at 298.15 K were measured and compared. Accordingly, the experimental binodal data were correlated using Merchuk, Hu, and Pirdashti equations, and the tie-line compositions were correlated using Othmer–Tobias and Bancroft equations. The effect of temperature, anion type, and length of alcohol chain on the binodal curve, tie-line length (TLL), slope of tie-line (STL), and phases’ physical properties were studied. It was found that an increase in salt’s hydrophobicity and alcohol’s carbon number in low temperature led to the expanding biphasic region and approaching the binodal curve to the origin. The phase-forming ability of different anions was studied using effective excluded volume (EEV) model, and the relation between the EEV value, saturation solubility, anion’s surface charge density, and salt solubility in water was investigated. It was found that kosmotropic ions with higher surface charge density and lower solubility in water have a higher EEV value and are able to form a wider two-phase area. Finally, the salting-out ability of different alcohols was compared with the Setschenow-type equation, and it was found that increasing the carbon number of alcohols enables the ATPS to salt out effectively.
中文翻译:

温度,盐和酒精的类型对2-丙醇+无机盐水溶液两相系统相图的影响:实验测定和相关性
测量了2-丙醇+磷酸三钾+水在298.15、308.15和318.15 K和2-丙醇+磷酸氢二钾+水在298.15 K时的液-液相平衡(LLE)。测量并比较了2-丙醇+硫酸钾/亚硫酸钾+水在298.15 K下的二元曲线。相应地,使用Merchuk,Hu和Pirdashti方程对实验的Biodal数据进行了关联,并且使用Othmer-Tobias和Bancroft方程对了联系线成分进行了关联。研究了温度,阴离子类型和醇链长度对双曲线曲线,连接线长度(TLL),连接线斜率(STL)和相的物理性质的影响。发现在低温下盐的疏水性和醇的碳原子数的增加导致双相区域的扩大,并接近双曲线曲线的原点。使用有效排量模型研究了不同阴离子的相形成能力,并研究了EEV值,饱和溶解度,阴离子表面电荷密度和盐在水中的溶解度之间的关系。已经发现,具有较高的表面电荷密度和较低的水溶解性的正离子具有较高的EEV值,并且能够形成较宽的两相区域。最后,将不同醇类的盐析能力与Setschenow型方程进行了比较,发现增加醇类的碳原子数可使ATPS有效地盐析。使用有效排量模型研究了不同阴离子的相形成能力,并研究了EEV值,饱和溶解度,阴离子表面电荷密度和盐在水中的溶解度之间的关系。已发现具有较高表面电荷密度和较低水溶解度的正离子具有较高的EEV值,并能够形成较宽的两相面积。最后,将不同醇类的盐析能力与Setschenow型方程进行了比较,发现增加醇类的碳原子数可使ATPS有效地盐析。使用有效排量模型研究了不同阴离子的相形成能力,并研究了EEV值,饱和溶解度,阴离子表面电荷密度和盐在水中的溶解度之间的关系。已发现具有较高表面电荷密度和较低水溶解度的正离子具有较高的EEV值,并能够形成较宽的两相面积。最后,将不同醇类的盐析能力与Setschenow型方程进行了比较,发现增加醇类的碳原子数可使ATPS有效地盐析。已发现具有较高表面电荷密度和较低水溶解度的正离子具有较高的EEV值,并能够形成较宽的两相面积。最后,将不同醇类的盐析能力与Setschenow型方程进行了比较,发现增加醇类的碳原子数可使ATPS有效地盐析。已发现具有较高表面电荷密度和较低水溶解度的正离子具有较高的EEV值,并能够形成较宽的两相面积。最后,将不同醇类的盐析能力与Setschenow型方程进行了比较,发现增加醇类的碳原子数可使ATPS有效地盐析。
更新日期:2018-07-08
中文翻译:

温度,盐和酒精的类型对2-丙醇+无机盐水溶液两相系统相图的影响:实验测定和相关性
测量了2-丙醇+磷酸三钾+水在298.15、308.15和318.15 K和2-丙醇+磷酸氢二钾+水在298.15 K时的液-液相平衡(LLE)。测量并比较了2-丙醇+硫酸钾/亚硫酸钾+水在298.15 K下的二元曲线。相应地,使用Merchuk,Hu和Pirdashti方程对实验的Biodal数据进行了关联,并且使用Othmer-Tobias和Bancroft方程对了联系线成分进行了关联。研究了温度,阴离子类型和醇链长度对双曲线曲线,连接线长度(TLL),连接线斜率(STL)和相的物理性质的影响。发现在低温下盐的疏水性和醇的碳原子数的增加导致双相区域的扩大,并接近双曲线曲线的原点。使用有效排量模型研究了不同阴离子的相形成能力,并研究了EEV值,饱和溶解度,阴离子表面电荷密度和盐在水中的溶解度之间的关系。已经发现,具有较高的表面电荷密度和较低的水溶解性的正离子具有较高的EEV值,并且能够形成较宽的两相区域。最后,将不同醇类的盐析能力与Setschenow型方程进行了比较,发现增加醇类的碳原子数可使ATPS有效地盐析。使用有效排量模型研究了不同阴离子的相形成能力,并研究了EEV值,饱和溶解度,阴离子表面电荷密度和盐在水中的溶解度之间的关系。已发现具有较高表面电荷密度和较低水溶解度的正离子具有较高的EEV值,并能够形成较宽的两相面积。最后,将不同醇类的盐析能力与Setschenow型方程进行了比较,发现增加醇类的碳原子数可使ATPS有效地盐析。使用有效排量模型研究了不同阴离子的相形成能力,并研究了EEV值,饱和溶解度,阴离子表面电荷密度和盐在水中的溶解度之间的关系。已发现具有较高表面电荷密度和较低水溶解度的正离子具有较高的EEV值,并能够形成较宽的两相面积。最后,将不同醇类的盐析能力与Setschenow型方程进行了比较,发现增加醇类的碳原子数可使ATPS有效地盐析。已发现具有较高表面电荷密度和较低水溶解度的正离子具有较高的EEV值,并能够形成较宽的两相面积。最后,将不同醇类的盐析能力与Setschenow型方程进行了比较,发现增加醇类的碳原子数可使ATPS有效地盐析。已发现具有较高表面电荷密度和较低水溶解度的正离子具有较高的EEV值,并能够形成较宽的两相面积。最后,将不同醇类的盐析能力与Setschenow型方程进行了比较,发现增加醇类的碳原子数可使ATPS有效地盐析。











































 京公网安备 11010802027423号
京公网安备 11010802027423号