当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Modeling Study toward Development of H2S-Free Removal of Iron Sulfide Scale from Oil and Gas Wells
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-07-19 , DOI: 10.1021/acs.iecr.8b01928 Wim Buijs 1 , Ibnelwaleed A. Hussein 2 , Mohamed Mahmoud 3 , Abdulmujeeb T. Onawole 2 , Mohammed A. Saad 4 , Golibjon R. Berdiyorov 5
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-07-19 , DOI: 10.1021/acs.iecr.8b01928 Wim Buijs 1 , Ibnelwaleed A. Hussein 2 , Mohamed Mahmoud 3 , Abdulmujeeb T. Onawole 2 , Mohammed A. Saad 4 , Golibjon R. Berdiyorov 5
Affiliation
|
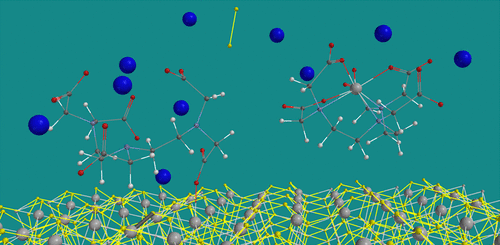
A common problem that faces the oil and gas industry is the formation of iron sulfide scale in various stages of production. Recently an effective chemical formulation was proposed to remove all types of iron sulfide scales (including pyrite), consisting of a chelating agent diethylenetriaminepentaacetic acid (DTPA) at high pH using potassium carbonate (K2CO3). The aim of this molecular modeling study is to develop insight into the thermodynamics and kinetics of the chemical reactions during scale removal. A cluster approach was chosen to mimic the overall system. Standard density functional theory (B3LYP/6-31G*) was used for all calculations. Low spin K4Fe(II)4(S2H)12 and K3Fe(II)(S2H)5 clusters were derived from the crystal structure of pyrite and used as mimics for surface scale FeS2. In addition, K5DTPA was used as a starting material too. High spin K3Fe(II)DTPA, and K2S2 were considered as products. A series of KmFe(II)(S2H)n complexes (m = n–2, n = 5–0) with various carboxylate and glycinate ligands was used to establish the most plausible reaction pathway. Some ligand exchange reactions were investigated on even simpler Fe(II) complexes in various spin states. It was found that the dissolution of iron sulfide scale with DTPA under basic conditions is thermodynamically favored and not limited by ligand exchange kinetics as the activation barriers for these reactions are very low. Singlet–quintet spin crossover and aqueous solvation of the products almost equally contribute to the overall reaction energy. Furthermore, seven-coordination to Fe(II) was observed in both high spin K3Fe(II)DTPA and K2Fe(II)(EDTA)(H2O) albeit in a slightly different manner.
中文翻译:

油气井中无H 2 S脱除硫化铁垢的分子模型研究
石油和天然气工业面临的一个普遍问题是在生产的各个阶段形成的硫化铁水垢。最近,有人提出了一种有效的化学配方,以去除所有类型的硫化铁垢(包括黄铁矿),其中包括使用碳酸钾(K 2 CO 3)在高pH下的螯合剂二亚乙基三胺五乙酸(DTPA )。这项分子模型研究的目的是深入研究除垢过程中化学反应的热力学和动力学。选择了集群方法来模仿整个系统。所有计算均使用标准密度泛函理论(B3LYP / 6-31G *)。低自旋K 4 Fe(II)4(S 2 H)12和K从黄铁矿的晶体结构衍生出3个Fe(II)(S 2 H)5团簇,并用作表面尺度FeS 2的模拟物。另外,K 5 DTPA也用作起始原料。高自旋的K 3 Fe(II)DTPA和K 2 S 2被认为是产物。一系列K m Fe(II)(S 2 H)n络合物(m = n –2,n= 5-0)具有各种羧酸盐和甘氨酸盐配体被用来建立最合理的反应途径。在各种自旋状态下,对甚至更简单的Fe(II)配合物也进行了一些配体交换反应的研究。发现在碱性条件下用DTPA溶解硫化铁水垢是热力学上有利的,并且不受配体交换动力学的限制,因为这些反应的活化势垒非常低。产品的单重态-五重态自旋交联和水溶剂化几乎平等地贡献了总反应能。此外,在高自旋K 3 Fe(II)DTPA和K 2 Fe(II)(EDTA)(H 2 O)中均观察到与Fe(II)的七配位,尽管方式略有不同。
更新日期:2018-07-20
中文翻译:

油气井中无H 2 S脱除硫化铁垢的分子模型研究
石油和天然气工业面临的一个普遍问题是在生产的各个阶段形成的硫化铁水垢。最近,有人提出了一种有效的化学配方,以去除所有类型的硫化铁垢(包括黄铁矿),其中包括使用碳酸钾(K 2 CO 3)在高pH下的螯合剂二亚乙基三胺五乙酸(DTPA )。这项分子模型研究的目的是深入研究除垢过程中化学反应的热力学和动力学。选择了集群方法来模仿整个系统。所有计算均使用标准密度泛函理论(B3LYP / 6-31G *)。低自旋K 4 Fe(II)4(S 2 H)12和K从黄铁矿的晶体结构衍生出3个Fe(II)(S 2 H)5团簇,并用作表面尺度FeS 2的模拟物。另外,K 5 DTPA也用作起始原料。高自旋的K 3 Fe(II)DTPA和K 2 S 2被认为是产物。一系列K m Fe(II)(S 2 H)n络合物(m = n –2,n= 5-0)具有各种羧酸盐和甘氨酸盐配体被用来建立最合理的反应途径。在各种自旋状态下,对甚至更简单的Fe(II)配合物也进行了一些配体交换反应的研究。发现在碱性条件下用DTPA溶解硫化铁水垢是热力学上有利的,并且不受配体交换动力学的限制,因为这些反应的活化势垒非常低。产品的单重态-五重态自旋交联和水溶剂化几乎平等地贡献了总反应能。此外,在高自旋K 3 Fe(II)DTPA和K 2 Fe(II)(EDTA)(H 2 O)中均观察到与Fe(II)的七配位,尽管方式略有不同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号