当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microkinetic Modeling and Reduced Rate Expression of the Water–Gas Shift Reaction on Nickel
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-07-18 , DOI: 10.1021/acs.iecr.8b01957 Thiago P. de Carvalho 1 , Rafael C. Catapan 1 , Amir A. M. Oliveira 2 , Dionisios G. Vlachos 3
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-07-18 , DOI: 10.1021/acs.iecr.8b01957 Thiago P. de Carvalho 1 , Rafael C. Catapan 1 , Amir A. M. Oliveira 2 , Dionisios G. Vlachos 3
Affiliation
|
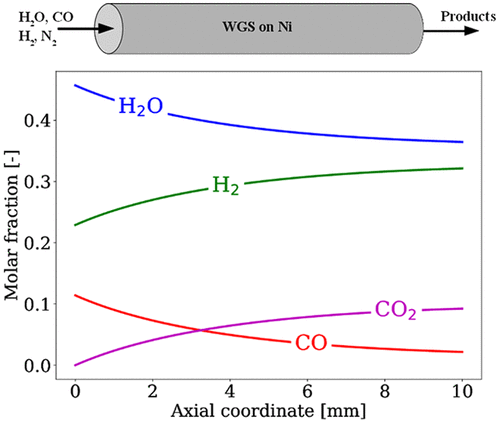
The development of a first-principles-based microkinetic modeling of the water–gas shift (WGS) reaction on nickel surfaces is presented. The surface reaction mechanism consists of 19 elementary reversible steps among 10 adsorbates. Density functional theory (DFT) was used to calculate the binding energies and transitions states of all adsorbates and reactions on Ni(111) and Ni(211) surfaces [Catapan ; DFT study of the water–gas shift reaction and coke formation on Ni (111) and Ni (211) surfaces. J. Phys. Chem. C 2012, 116, 20281−20291]. Thermodynamic consistency of the DFT-predicted energetics was taken into account in the construction of the kinetic mechanism. Lateral interactions between adsorbates were calculated via DFT and included in the microkinetic modeling using a hierarchical approach. The model predictions compare well with experimental results on Ni/Al2O3 reported in the literature, reproducing CO conversion, apparent activation energy, and reaction orders for CO and H2O. A reduced microkinetic model is derived using sensitivity and principal component analysis. The main reaction pathway analysis revealed that the carboxyl pathway is favored and the elementary step CO* + OH* ⇌ COOH* + * is the rate-determining step of WGS on Ni. A global one-step rate expression for WGS on Ni was also developed. Moreover, a novel thermodynamic-consistent treatment for the evaluation species coverages in the rate expression is proposed. This came with the use of a look-up table for the most abundant adsorbed species and has improved the performance of the one-step expression over a wide range of conditions (inlet compositions, volumetric flow rates, and different temperatures).
中文翻译:

镍上水煤气变换反应的微观动力学模型和降低的速率表达
提出了基于第一原理的镍表面水煤气变换(WGS)反应微动力学模型的开发。表面反应机理由10种吸附物中的19个基本可逆步骤组成。密度泛函理论(DFT)用于计算Ni(111)和Ni(211)表面上所有吸附物的键能和过渡态以及反应[卡塔潘 ; DFT研究了Ni(111)和Ni(211)表面的水煤气变换反应和焦炭形成。J.物理 化学 Ç 2012,116,20281-20291]。DFT预测的能量学的热力学一致性在动力学机理的构建中已被考虑在内。通过DFT计算吸附物之间的横向相互作用,并使用分层方法将其包括在微动力学模型中。该模型预测与文献中报道的关于Ni / Al 2 O 3的实验结果很好地比较,再现了CO转化率,表观活化能以及CO和H 2的反应顺序。O.使用敏感性和主成分分析得出简化的微动力学模型。主要的反应路径分析表明,羧基路径是有利的,基本步骤CO * + OH *⇌COOH * + *是WGS在Ni上的速率决定步骤。还开发了一种针对Ni的WGS的全局单步速率表达式。此外,提出了一种新的热力学一致性处理方法,用于评价速率表达中的物种覆盖率。这是通过使用查找表来获取最丰富的吸附物质,并在很大范围的条件(进样口组成,体积流量和不同温度)下提高了一步表达的性能。
更新日期:2018-07-19
中文翻译:

镍上水煤气变换反应的微观动力学模型和降低的速率表达
提出了基于第一原理的镍表面水煤气变换(WGS)反应微动力学模型的开发。表面反应机理由10种吸附物中的19个基本可逆步骤组成。密度泛函理论(DFT)用于计算Ni(111)和Ni(211)表面上所有吸附物的键能和过渡态以及反应[











































 京公网安备 11010802027423号
京公网安备 11010802027423号