当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
MXene nanoribbons as electrocatalysts for the hydrogen evolution reaction with fast kinetics†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1039/c8cp02635a Xiaowei Yang 1, 2, 3, 4, 5 , Nan Gao 1, 2, 3, 4, 5 , Si Zhou 1, 2, 3, 4, 5 , Jijun Zhao 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1039/c8cp02635a Xiaowei Yang 1, 2, 3, 4, 5 , Nan Gao 1, 2, 3, 4, 5 , Si Zhou 1, 2, 3, 4, 5 , Jijun Zhao 1, 2, 3, 4, 5
Affiliation
|
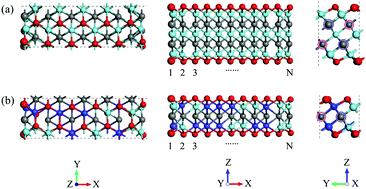
MXenes, a new class of two-dimensional materials, arouse great interest due to their diverse chemistries, superior electrical conductivity and stability. Recently, the nanostructures of MXenes such as nanoribbons and nanodots have been synthesized in experiments, which show peculiar properties and expand the application spectrum of MXenes. Here we exploited MXene nanoribbons as potential electrocatalysts for the hydrogen evolution reaction (HER) by considering 12 kinds of MXene systems. Our first-principles calculations showed that the edges of the MXene nanoribbons can adsorb hydrogen species and serve as the reaction sites for hydrogen evolution. The binding strength of the ribbon edge is correlated with the d band center of metal atoms in MXenes. In particular, the nanoribbons of Ti3C2 and solid solution (Ti,Nb)C exhibit high activity for the HER with the adsorption free energy approaching zero and Tafel barrier below 0.42 and 0.17 eV, respectively. The low barrier is owing to the prominent charge transfer from the edge metal atoms to the H* reactants in the transition state. These theoretical results illuminate the principle for designing MXene nanostructures for electrocatalysts with fast kinetics, and shed light on the utilization of MXenes with more than one metal element for a broad range of electrochemical reactions.
中文翻译:

MXene纳米带作为快速动力学的氢释放反应的电催化剂†
MXenes是一类新型的二维材料,由于其多样的化学性质,出色的导电性和稳定性而引起了人们的极大兴趣。近来,已经通过实验合成了MXenes的纳米结构,例如纳米带和纳米点,其表现出独特的性能并扩展了MXenes的应用范围。在这里,我们通过考虑12种MXene系统,将MXene纳米带用作潜在的氢析出反应(HER)的电催化剂。我们的第一性原理计算表明,MXene纳米带的边缘可以吸附氢物种,并充当氢释放的反应位点。碳带边缘的结合强度与MXenes中金属原子的d能带中心相关。特别是Ti 3 C 2的纳米带固溶体(Ti,Nb)C对HER表现出较高的活性,其吸附自由能分别接近零和Tafel势垒,分别低于0.42和0.17 eV。低势垒是由于在过渡态中从边缘金属原子到H *反应物的显着电荷转移所致。这些理论结果阐明了设计具有快速动力学的电催化剂的MXene纳米结构的原理,并阐明了具有多种金属元素的MXene在广泛的电化学反应中的应用。
更新日期:2018-07-06
中文翻译:

MXene纳米带作为快速动力学的氢释放反应的电催化剂†
MXenes是一类新型的二维材料,由于其多样的化学性质,出色的导电性和稳定性而引起了人们的极大兴趣。近来,已经通过实验合成了MXenes的纳米结构,例如纳米带和纳米点,其表现出独特的性能并扩展了MXenes的应用范围。在这里,我们通过考虑12种MXene系统,将MXene纳米带用作潜在的氢析出反应(HER)的电催化剂。我们的第一性原理计算表明,MXene纳米带的边缘可以吸附氢物种,并充当氢释放的反应位点。碳带边缘的结合强度与MXenes中金属原子的d能带中心相关。特别是Ti 3 C 2的纳米带固溶体(Ti,Nb)C对HER表现出较高的活性,其吸附自由能分别接近零和Tafel势垒,分别低于0.42和0.17 eV。低势垒是由于在过渡态中从边缘金属原子到H *反应物的显着电荷转移所致。这些理论结果阐明了设计具有快速动力学的电催化剂的MXene纳米结构的原理,并阐明了具有多种金属元素的MXene在广泛的电化学反应中的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号