当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Prediction of disulfide dihedral angles using chemical shifts†
Chemical Science ( IF 7.6 ) Pub Date : 2018-07-05 00:00:00 , DOI: 10.1039/c8sc01423j David A Armstrong 1 , Quentin Kaas 2 , K Johan Rosengren 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-07-05 00:00:00 , DOI: 10.1039/c8sc01423j David A Armstrong 1 , Quentin Kaas 2 , K Johan Rosengren 1
Affiliation
|
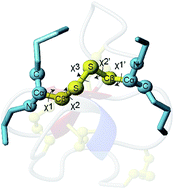
Cystine residues result from the formation of disulfide bonds between pairs of cysteine residues. This cross linking of the backbone is essential for the structure and activity of peptides and proteins. The conformation of a cystine side chain can be described using five dihedral angles, χ1, χ2, χ3, χ2′, and χ1′, with cystines favouring certain combinations of these angles. 2D NMR spectroscopy is ideally suited for structure determination of disulfide-rich peptides, because of their small size and constrained nature. However, only limited information of the cystine side chain conformation can be determined by NMR spectroscopy, leading to ambiguity in the deduced 3D structures. Resolving accurate structures is important as disulfide-rich peptides have proven to be promising drug candidates in a number of fields, either as bioactive leads or scaffolds. Using a database of NMR chemical shifts combined with crystallographic structures, we have developed a method called DISH that uses support vector machines to predict the dihedral angles of cysteine side chains. It is able to successfully predict χ2 angles with 91% accuracy, and has improved performance over existing prediction methods for χ1 angles, with 87% accuracy. For 81% of cysteine residues, DISH successfully predicted both the χ1 and χ2 angles. By revisiting published solution structures of peptides determined using NMR spectroscopy, we assessed the impact of additional cystine dihedral restraints on the quality of 3D models. DISH improved the resolution and accuracy, highlighting the potential for improving the understanding of structure–activity relationships and rational development of peptide drugs.
中文翻译:

使用化学位移预测二硫键二面角†
半胱氨酸残基是由半胱氨酸残基对之间形成二硫键产生的。主链的这种交联对于肽和蛋白质的结构和活性至关重要。胱氨酸侧链的构象可以使用五个二面角χ 1 、χ 2 、χ 3 、χ 2' 和χ 1' 来描述,其中胱氨酸倾向于这些角度的某些组合。二维核磁共振波谱非常适合富含二硫键的肽的结构测定,因为它们尺寸小且性质有限。然而,NMR 波谱只能确定胱氨酸侧链构象的有限信息,导致推导的 3D 结构不明确。解析准确的结构非常重要,因为富含二硫键的肽已被证明是许多领域有前途的候选药物,无论是作为生物活性先导化合物还是支架。利用 NMR 化学位移数据库与晶体结构相结合,我们开发了一种称为 DISH 的方法,该方法使用支持向量机来预测半胱氨酸侧链的二面角。它能够以 91% 的准确率成功预测χ 2 角度,并且比现有的χ 1 角度预测方法提高了性能,准确率达到 87%。对于 81% 的半胱氨酸残基,DISH 成功预测了χ 1 和χ 2 角度。通过重新审视已发表的使用 NMR 波谱测定的肽溶液结构,我们评估了额外的胱氨酸二面体限制对 3D 模型质量的影响。DISH 提高了分辨率和准确性,凸显了提高对结构-活性关系的理解和肽药物合理开发的潜力。
更新日期:2018-07-05
中文翻译:

使用化学位移预测二硫键二面角†
半胱氨酸残基是由半胱氨酸残基对之间形成二硫键产生的。主链的这种交联对于肽和蛋白质的结构和活性至关重要。胱氨酸侧链的构象可以使用五个二面角χ 1 、χ 2 、χ 3 、χ 2' 和χ 1' 来描述,其中胱氨酸倾向于这些角度的某些组合。二维核磁共振波谱非常适合富含二硫键的肽的结构测定,因为它们尺寸小且性质有限。然而,NMR 波谱只能确定胱氨酸侧链构象的有限信息,导致推导的 3D 结构不明确。解析准确的结构非常重要,因为富含二硫键的肽已被证明是许多领域有前途的候选药物,无论是作为生物活性先导化合物还是支架。利用 NMR 化学位移数据库与晶体结构相结合,我们开发了一种称为 DISH 的方法,该方法使用支持向量机来预测半胱氨酸侧链的二面角。它能够以 91% 的准确率成功预测χ 2 角度,并且比现有的χ 1 角度预测方法提高了性能,准确率达到 87%。对于 81% 的半胱氨酸残基,DISH 成功预测了χ 1 和χ 2 角度。通过重新审视已发表的使用 NMR 波谱测定的肽溶液结构,我们评估了额外的胱氨酸二面体限制对 3D 模型质量的影响。DISH 提高了分辨率和准确性,凸显了提高对结构-活性关系的理解和肽药物合理开发的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号