当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Energy Storage in Poly(dithieno[3,2-b:2′,3′-d]pyrrole) Bearing Pendant Nitroxide Radicals
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-07-05 00:00:00 , DOI: 10.1021/acs.chemmater.8b01775 Fei Li , Shaoyang Wang , Yanpu Zhang , Jodie L. Lutkenhaus
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-07-05 00:00:00 , DOI: 10.1021/acs.chemmater.8b01775 Fei Li , Shaoyang Wang , Yanpu Zhang , Jodie L. Lutkenhaus
|
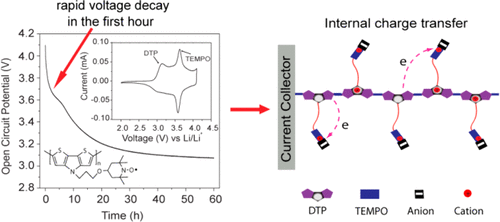
The design and electrochemical synthesis of a conjugated radical polymer (CRP), poly(dithieno[3,2-b:2′,3′-d]pyrrole) bearing pendant nitroxide radicals, is reported. Conjugated radical polymers potentially offer simultaneous conductivity and redox activity in the context of organic energy storage. One challenge is understanding the internal electron transfer that occurs in CRPs, which affects the electrochemical energy storage properties. The CRP here is purposefully designed to examine the case of when the conjugated backbone’s redox potential is less than that of the organic radical group. Cyclic voltammetry on the as-prepared CRP exhibits two well-resolved redox peaks at Epa1 = 3.15 V and Epa2 = 3.61 V vs Li/Li+, corresponding to the redox activities of the (dithieno[3,2-b:2′,3′-d]pyrrole) (DTP) backbone and nitroxide radical, respectively. Galvanostatic charge/discharge studies also reveal a two-step charge/discharge process. The lower oxidation potential of DTP contributes to a conductive pathway during the charge/discharge process. An internal electron transfer process occurs during the decay of the open circuit potential, as the final potential stabilizes around 3 V. This strategy emphasizes the effects on energy storage when the redox active polymer contains two moieties that are redox active at different potentials, thus impacting future CRP design.
中文翻译:

聚(二硫代[3,2-b:2',3'-d]吡咯)侧链一氧化氮自由基中的电化学储能
报道了带有侧基氮氧化物自由基的共轭自由基聚合物(CRP)的设计和电化学合成,聚(二硫代[3,2-b:2',3'-d]吡咯)。在有机能量存储的情况下,共轭自由基聚合物可能同时提供导电性和氧化还原活性。挑战之一是了解CRP中发生的内部电子转移,这会影响电化学储能性能。此处的CRP专门用于检查共轭主链的氧化还原电势小于有机基团的氧化还原电势的情况。与Li / Li +相比,制备的CRP的循环伏安法在E pa1 = 3.15 V和E pa2 = 3.61 V处显示两个分辨良好的氧化还原峰。分别对应于(dithieno [3,2-b:2',3'-d]吡咯)(DTP)骨架和一氧化氮自由基的氧化还原活性。恒电流充电/放电研究还揭示了两步充电/放电过程。DTP的较低氧化电势在充电/放电过程中有助于导电路径。内部电子传输过程在开路电势衰减期间发生,因为最终电势稳定在3 V左右。当氧化还原活性聚合物包含两个在不同电势下具有氧化还原活性的部分时,该策略强调了对能量存储的影响。未来的CRP设计。
更新日期:2018-07-05
中文翻译:

聚(二硫代[3,2-b:2',3'-d]吡咯)侧链一氧化氮自由基中的电化学储能
报道了带有侧基氮氧化物自由基的共轭自由基聚合物(CRP)的设计和电化学合成,聚(二硫代[3,2-b:2',3'-d]吡咯)。在有机能量存储的情况下,共轭自由基聚合物可能同时提供导电性和氧化还原活性。挑战之一是了解CRP中发生的内部电子转移,这会影响电化学储能性能。此处的CRP专门用于检查共轭主链的氧化还原电势小于有机基团的氧化还原电势的情况。与Li / Li +相比,制备的CRP的循环伏安法在E pa1 = 3.15 V和E pa2 = 3.61 V处显示两个分辨良好的氧化还原峰。分别对应于(dithieno [3,2-b:2',3'-d]吡咯)(DTP)骨架和一氧化氮自由基的氧化还原活性。恒电流充电/放电研究还揭示了两步充电/放电过程。DTP的较低氧化电势在充电/放电过程中有助于导电路径。内部电子传输过程在开路电势衰减期间发生,因为最终电势稳定在3 V左右。当氧化还原活性聚合物包含两个在不同电势下具有氧化还原活性的部分时,该策略强调了对能量存储的影响。未来的CRP设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号