当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
d-Amino Acid-Mediated Translation Arrest Is Modulated by the Identity of the Incoming Aminoacyl-tRNA
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1021/acs.biochem.8b00595 Rachel C. Fleisher 1 , Virginia W. Cornish 1 , Ruben L. Gonzalez 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1021/acs.biochem.8b00595 Rachel C. Fleisher 1 , Virginia W. Cornish 1 , Ruben L. Gonzalez 1
Affiliation
|
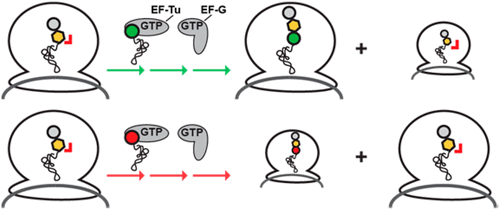
A complete understanding of the determinants that restrict d-amino acid incorporation by the ribosome, which is of interest to both basic biologists and the protein engineering community, remains elusive. Previously, we demonstrated that d-amino acids are successfully incorporated into the C-terminus of the nascent polypeptide chain. Ribosomes carrying the resulting peptidyl-d-aminoacyl-tRNA (peptidyl-d-aa-tRNA) donor substrate, however, partition into subpopulations that either undergo translation arrest through inactivation of the ribosomal peptidyl-transferase center (PTC) or remain translationally competent. The proportion of each subpopulation is determined by the identity of the d-amino acid side chain. Here, we demonstrate that the identity of the aminoacyl-tRNA (aa-tRNA) acceptor substrate that is delivered to ribosomes carrying a peptidyl-d-aa-tRNA donor further modulates this partitioning. Our discovery demonstrates that it is the pairing of the peptidyl-d-aa-tRNA donor and the aa-tRNA acceptor that determines the activity of the PTC. Moreover, we provide evidence that both the amino acid and tRNA components of the aa-tRNA acceptor contribute synergistically to the extent of arrest. The results of this work deepen our understanding of the mechanism of d-amino acid-mediated translation arrest and how cells avoid this precarious obstacle, reveal similarities to other translation arrest mechanisms involving the PTC, and provide a new route for improving the yields of engineered proteins containing d-amino acids.
中文翻译:

d-氨基酸介导的翻译逮捕是由传入的氨酰基-tRNA的身份进行调制。
基本生物学家和蛋白质工程界都对使核糖体限制d-氨基酸掺入的决定因素有一个完整的了解。以前,我们证明了d-氨基酸已成功整合到新生多肽链的C末端。携带核糖体所得到的肽基d氨基酰基酰tRNA(肽基d -aa酰tRNA)施主衬底,但是,分区成既可以通过核糖体肽基转移酶中心(PTC)的失活经历翻译停滞或保持平移主管亚群。每个子群体的比例由d的身份确定-氨基酸侧链。在这里,我们证明了氨基酰基-tRNA(aa-tRNA)受体底物的身份被传递到携带肽基-d -aa-tRNA供体的核糖体中,进一步调节了这种分配。我们的发现表明,决定PTC活性的是肽基-d -aa-tRNA供体与aa-tRNA受体的配对。此外,我们提供的证据表明,aa-tRNA受体的氨基酸和tRNA成分在停滞程度上均起协同作用。这项工作的结果加深了我们对d机理的理解。-氨基酸介导的翻译阻滞作用以及细胞如何避免这种不稳定的障碍,揭示了与涉及PTC的其他翻译阻滞机制的相似性,并为提高含d-氨基酸的工程蛋白的产量提供了一条新途径。
更新日期:2018-07-06
中文翻译:

d-氨基酸介导的翻译逮捕是由传入的氨酰基-tRNA的身份进行调制。
基本生物学家和蛋白质工程界都对使核糖体限制d-氨基酸掺入的决定因素有一个完整的了解。以前,我们证明了d-氨基酸已成功整合到新生多肽链的C末端。携带核糖体所得到的肽基d氨基酰基酰tRNA(肽基d -aa酰tRNA)施主衬底,但是,分区成既可以通过核糖体肽基转移酶中心(PTC)的失活经历翻译停滞或保持平移主管亚群。每个子群体的比例由d的身份确定-氨基酸侧链。在这里,我们证明了氨基酰基-tRNA(aa-tRNA)受体底物的身份被传递到携带肽基-d -aa-tRNA供体的核糖体中,进一步调节了这种分配。我们的发现表明,决定PTC活性的是肽基-d -aa-tRNA供体与aa-tRNA受体的配对。此外,我们提供的证据表明,aa-tRNA受体的氨基酸和tRNA成分在停滞程度上均起协同作用。这项工作的结果加深了我们对d机理的理解。-氨基酸介导的翻译阻滞作用以及细胞如何避免这种不稳定的障碍,揭示了与涉及PTC的其他翻译阻滞机制的相似性,并为提高含d-氨基酸的工程蛋白的产量提供了一条新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号