当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Serine/Threonine Ligation: Origin, Mechanistic Aspects, and Applications
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1021/acs.accounts.8b00151 Han Liu 1 , Xuechen Li 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-07-06 00:00:00 , DOI: 10.1021/acs.accounts.8b00151 Han Liu 1 , Xuechen Li 1
Affiliation
|
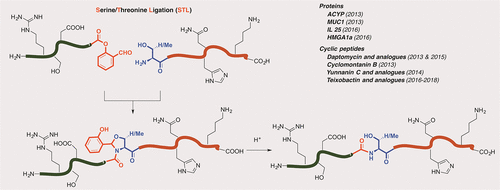
Synthetic proteins are expected to go beyond the boundary of recombinant DNA expression systems by being flexibly installed with site-specific natural or unnatural modification structures during synthesis. To enable protein chemical synthesis, peptide ligations provide effective strategies to assemble short peptide fragments obtained from solid-phase peptide synthesis (SPPS) into long peptides and proteins. In this regard, chemoselective peptide ligation represents a simple but powerful transformation realizing selective amide formation between the C-terminus and N-terminus of two side-chain-unprotected peptide fragments. These reactions are highly chemo- and regioselective to tolerate the side-chain functionalities present on the unprotected peptides, highly reactive to work with millmolar or submillimolar concentrations of the substrates, and operationally simple with mild conditions and accessible building blocks.
中文翻译:

丝氨酸/苏氨酸连接:起源,机制方面和应用。
通过在合成过程中灵活地安装具有位点特异性的天然或非天然修饰结构,预计合成蛋白将超出重组DNA表达系统的范围。为了实现蛋白质化学合成,肽段连接提供了有效的策略,可将从固相肽合成(SPPS)获得的短肽片段组装为长肽和蛋白质。就这一点而言,化学选择性肽的连接代表了一个简单但功能强大的转化,实现了两个侧链未保护的肽片段的C端和N端之间的选择性酰胺形成。这些反应具有高度的化学选择性和区域选择性,可以耐受未保护肽上存在的侧链功能,对以毫摩尔或亚毫摩尔浓度的底物起作用时具有高反应性,
更新日期:2018-07-06
中文翻译:

丝氨酸/苏氨酸连接:起源,机制方面和应用。
通过在合成过程中灵活地安装具有位点特异性的天然或非天然修饰结构,预计合成蛋白将超出重组DNA表达系统的范围。为了实现蛋白质化学合成,肽段连接提供了有效的策略,可将从固相肽合成(SPPS)获得的短肽片段组装为长肽和蛋白质。就这一点而言,化学选择性肽的连接代表了一个简单但功能强大的转化,实现了两个侧链未保护的肽片段的C端和N端之间的选择性酰胺形成。这些反应具有高度的化学选择性和区域选择性,可以耐受未保护肽上存在的侧链功能,对以毫摩尔或亚毫摩尔浓度的底物起作用时具有高反应性,











































 京公网安备 11010802027423号
京公网安备 11010802027423号