当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Successfully Engineering a Bacterial Sialyltransferase for Regioselective α2,6-sialylation
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-07-05 00:00:00 , DOI: 10.1021/acscatal.8b01993 Yangyang Xu 1 , Yueyuan Fan 1 , Jinfeng Ye 2 , Faxing Wang 1 , Quandeng Nie 1 , Li Wang 1 , Peng George Wang 1 , Hongzhi Cao 2 , Jiansong Cheng 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-07-05 00:00:00 , DOI: 10.1021/acscatal.8b01993 Yangyang Xu 1 , Yueyuan Fan 1 , Jinfeng Ye 2 , Faxing Wang 1 , Quandeng Nie 1 , Li Wang 1 , Peng George Wang 1 , Hongzhi Cao 2 , Jiansong Cheng 1
Affiliation
|
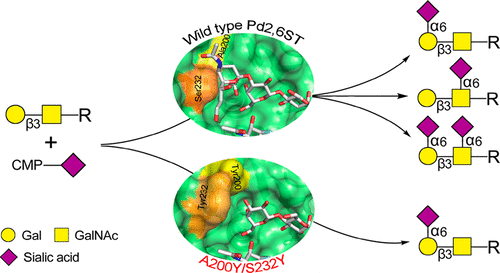
A β-galactoside α2,6-sialyltransferase from Photobacterium damselae (Pd2,6ST) that is capable of sialylating both terminal and internal galactose and N-acetylgalactosamine was herein redesigned for regioselectively producing terminal α2,6-sialosides. Guided by a recently developed bump-hole strategy, a series of mutations at Ala200 and Ser232 sites were created for reshaping the acceptor binding pocket. Finally, a Pd2,6ST double mutant A200Y/S232Y with an altered L-shaped acceptor binding pocket was identified to be a superior α2,6-sialyltransferase which can efficiently catalyze the regioselective α2,6-sialylation of galactose or N-acetylgalactosamine at the nonreducing end of a series of glycans. Meanwhile, A200Y/S232Y remains flexible donor substrate specificity and is able to transfer Neu5Ac, Neu5Gc, and KDN.
中文翻译:

成功设计出细菌唾液酸转移酶用于区域选择性α2,6-唾液酸化
本文重新设计了能够使末端半乳糖和内部半乳糖以及N-乙酰半乳糖胺唾液酸化的来自damselae的细菌的β-半乳糖苷α2,6-唾液酸转移酶(Pd2,6ST),在区域上选择性地产生末端α2,6-唾液酸。在最近开发的撞孔策略的指导下,在Ala200和Ser232位点产生了一系列突变,以重塑受体结合口袋。最后,鉴定出具有改变的L形受体结合口袋的Pd2,6ST双突变体A200Y / S232Y是优良的α2,6-唾液酸转移酶,其可以有效地催化半乳糖或N的区域选择性α2,6-唾液酸化。-乙酰半乳糖胺在一系列聚糖的非还原端。同时,A200Y / S232Y保持灵活的供体底物特异性,并能够转移Neu5Ac,Neu5Gc和KDN。
更新日期:2018-07-05
中文翻译:

成功设计出细菌唾液酸转移酶用于区域选择性α2,6-唾液酸化
本文重新设计了能够使末端半乳糖和内部半乳糖以及N-乙酰半乳糖胺唾液酸化的来自damselae的细菌的β-半乳糖苷α2,6-唾液酸转移酶(Pd2,6ST),在区域上选择性地产生末端α2,6-唾液酸。在最近开发的撞孔策略的指导下,在Ala200和Ser232位点产生了一系列突变,以重塑受体结合口袋。最后,鉴定出具有改变的L形受体结合口袋的Pd2,6ST双突变体A200Y / S232Y是优良的α2,6-唾液酸转移酶,其可以有效地催化半乳糖或N的区域选择性α2,6-唾液酸化。-乙酰半乳糖胺在一系列聚糖的非还原端。同时,A200Y / S232Y保持灵活的供体底物特异性,并能够转移Neu5Ac,Neu5Gc和KDN。











































 京公网安备 11010802027423号
京公网安备 11010802027423号