Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural insights into G-protein-coupled receptor allostery
Nature ( IF 50.5 ) Pub Date : 2018-07-01 , DOI: 10.1038/s41586-018-0259-z David M. Thal , Alisa Glukhova , Patrick M. Sexton , Arthur Christopoulos
Nature ( IF 50.5 ) Pub Date : 2018-07-01 , DOI: 10.1038/s41586-018-0259-z David M. Thal , Alisa Glukhova , Patrick M. Sexton , Arthur Christopoulos
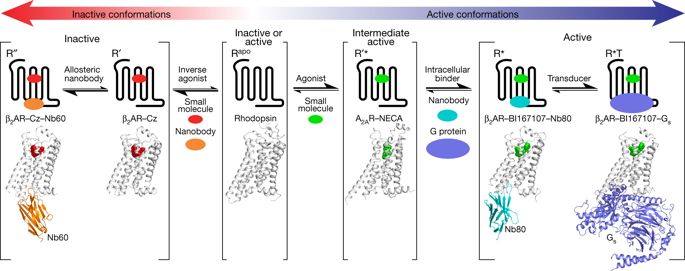
|
G-protein-coupled receptors (GPCRs) are key cell-surface proteins that transduce external environmental cues into biochemical signals across the membrane. GPCRs are intrinsically allosteric proteins; they interact via spatially distinct yet conformationally linked domains with both endogenous and exogenous proteins, nutrients, metabolites, hormones, small molecules and biological agents. Here we explore recent high-resolution structural studies, which are beginning to unravel the atomic details of allosteric transitions that govern GPCR biology, as well as highlighting how the wide diversity of druggable allosteric sites across these receptors present opportunities for developing new classes of therapeutics.High-resolution structural studies of GPCRs have led to insights into the role of allostery in GPCR-mediated signal transduction.
中文翻译:

G蛋白偶联受体变构的结构洞察
G 蛋白偶联受体 (GPCR) 是关键的细胞表面蛋白,可将外部环境信号转导为跨膜生化信号。GPCR 本质上是变构蛋白;它们通过空间不同但构象相连的结构域与内源性和外源性蛋白质、营养素、代谢物、激素、小分子和生物制剂相互作用。在这里,我们探索了最近的高分辨率结构研究,这些研究开始揭示控制 GPCR 生物学的变构转变的原子细节,并强调这些受体中可药物变构位点的广泛多样性如何为开发新的治疗方法提供机会。 GPCR 的高分辨率结构研究使人们深入了解变构在 GPCR 介导的信号转导中的作用。
更新日期:2018-07-01
中文翻译:

G蛋白偶联受体变构的结构洞察
G 蛋白偶联受体 (GPCR) 是关键的细胞表面蛋白,可将外部环境信号转导为跨膜生化信号。GPCR 本质上是变构蛋白;它们通过空间不同但构象相连的结构域与内源性和外源性蛋白质、营养素、代谢物、激素、小分子和生物制剂相互作用。在这里,我们探索了最近的高分辨率结构研究,这些研究开始揭示控制 GPCR 生物学的变构转变的原子细节,并强调这些受体中可药物变构位点的广泛多样性如何为开发新的治疗方法提供机会。 GPCR 的高分辨率结构研究使人们深入了解变构在 GPCR 介导的信号转导中的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号