当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed carbonylation of thioacetates and aryl iodides for the synthesis of S-aryl thioesters†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-07-04 00:00:00 , DOI: 10.1039/c8qo00466h Myungjin Kim 1, 2, 3, 4 , Subeen Yu 1, 2, 3, 4 , Jeung Gon Kim 1, 4, 5, 6 , Sunwoo Lee 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-07-04 00:00:00 , DOI: 10.1039/c8qo00466h Myungjin Kim 1, 2, 3, 4 , Subeen Yu 1, 2, 3, 4 , Jeung Gon Kim 1, 4, 5, 6 , Sunwoo Lee 1, 2, 3, 4
Affiliation
|
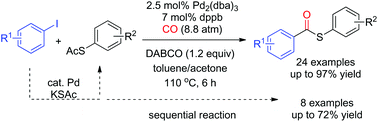
Thioesters were synthesized via palladium-catalyzed carbonylation of thioacetates and aryl iodides. S-Aryl thioacetates coupled with carbon monoxide and aryl iodides to afford the desired S-aryl thioesters in good yields. The reaction showed good functional group tolerance toward fluoro, chloro, ketone, ester, aldehyde, cyano, and nitro groups. The tandem reaction of the direct S-arylation of aryl iodides from potassium thioacetate (KSAc) and subsequent carbonylation of the intermediates S-aryl thioacetates provided S-aryl thioesters in moderate-to-good yields.
中文翻译:

钯催化的硫代乙酸盐和芳基碘化物的羰基化反应,用于合成S-芳基硫酯†
硫酯是通过钯催化的硫代乙酸酯和芳基碘化物的羰基化反应合成的。S-芳基硫代乙酸酯与一氧化碳和芳基碘化物偶联,以高收率提供所需的S-芳基硫代酯。反应显示出对氟,氯,酮,酯,醛,氰基和硝基的良好的官能团耐受性。来自硫代乙酸钾(KSAc)的芳基碘化物的直接S-芳基化和中间体S-芳基硫代乙酸酯的后续羰基化的串联反应以中等至良好的收率提供了S-芳基硫代酯。
更新日期:2018-07-04
中文翻译:

钯催化的硫代乙酸盐和芳基碘化物的羰基化反应,用于合成S-芳基硫酯†
硫酯是通过钯催化的硫代乙酸酯和芳基碘化物的羰基化反应合成的。S-芳基硫代乙酸酯与一氧化碳和芳基碘化物偶联,以高收率提供所需的S-芳基硫代酯。反应显示出对氟,氯,酮,酯,醛,氰基和硝基的良好的官能团耐受性。来自硫代乙酸钾(KSAc)的芳基碘化物的直接S-芳基化和中间体S-芳基硫代乙酸酯的后续羰基化的串联反应以中等至良好的收率提供了S-芳基硫代酯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号