当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Putative metal binding site in the transmembrane domain of the manganese transporter SLC30A10 is different from that of related zinc transporters
Metallomics ( IF 2.9 ) Pub Date : 2018-07-04 00:00:00 , DOI: 10.1039/c8mt00115d Charles E. Zogzas 1, 2, 3, 4, 5 , Somshuvra Mukhopadhyay 1, 2, 3, 4, 5
Metallomics ( IF 2.9 ) Pub Date : 2018-07-04 00:00:00 , DOI: 10.1039/c8mt00115d Charles E. Zogzas 1, 2, 3, 4, 5 , Somshuvra Mukhopadhyay 1, 2, 3, 4, 5
Affiliation
|
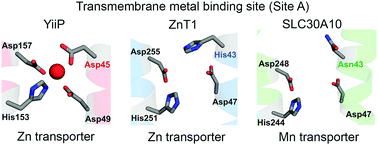
SLC30 proteins belong to the cation diffusion facilitator (CDF) superfamily of metal transporters. SLC30A10 mediates manganese efflux, while other SLC30 members transport zinc. Metal specificity of CDFs may be conferred by amino acids that form a transmembrane metal binding site (Site A). Site A of zinc-transporting CDFs, such as SLC30A1/ZnT1, have a HXXXD motif, but manganese transporters, such as SLC30A10, harbor a NXXXD motif. This critical histidine-to-asparagine substitution, at residue 43, was proposed to underlie manganese transport specificity of SLC30A10. However, we recently discovered that asparagine-43 was dispensable for manganese efflux in HeLa cells; instead, glutamate-25, aspartate-40, asparagine-127, and aspartate-248 were required. In contrast, another group reported that asparagine-43 was required in a chicken cell line. The goal of this study was to resolve the divergent results about the requirement of the crucial asparagine-43 residue. For this, we compared the manganese efflux activity of four cell types that stably over-expressed SLC30A10wild-type (WT), SLC30A10N43A or SLC30A10E25A: physiologically-relevant hepatic HepG2 and neuronal AF5 cells, HEK cells, and embryonic fibroblasts from Slc30a10−/− mice. In all cell types, manganese efflux activity of SLC30A10N43A was comparable to WT, while SLC30A10E25A lacked activity. Importantly, unlike SLC30A10, the histidine residue of the HXXXD motif of SLC30A1/ZnT1 was required for zinc transport. These results imply that the mechanisms of ion coordination within the transmembrane domain of SLC30A10 substantially differ from previously-studied CDFs, suggest that factors beyond Site A residues may confer metal specificity to CDFs, and improve understanding of the pathobiology of manganese toxicity due to mutations in SLC30A10.
中文翻译:

锰转运蛋白SLC30A10跨膜结构域的推定金属结合位点与相关的锌转运蛋白不同
SLC30蛋白属于金属转运蛋白的阳离子扩散促进子(CDF)超家族。SLC30A10介导锰流出,而其他SLC30成员则输送锌。CDF的金属特异性可以由形成跨膜金属结合位点的氨基酸赋予(部位A)。含锌的CDF的位点A(例如SLC30A1 / ZnT1)具有HXXXD图案,但是锰转运蛋白(例如SLC30A10)带有NXXXD主题。有人提出在残基43处进行此关键的组氨酸至天冬酰胺关键取代是SLC30A10锰转运特异性的基础。然而,我们最近发现,天冬酰胺43对于HeLa细胞中的锰流出是不可或缺的。相反,需要谷氨酸25,天冬氨酸40,天冬酰胺127和天冬氨酸248。相反,另一小组报告说,鸡细胞系中需要天冬酰胺43。这项研究的目的是解决关于关键天冬酰胺43残留量需求的分歧结果。为此,我们比较了稳定过量表达SLC30A10野生型(WT),SLC30A10 N43A或SLC30A10 E25A的四种细胞类型的锰外流活性:来自Slc30a10 - /-小鼠的与生理相关的肝HepG2和神经元AF5细胞,HEK细胞以及胚胎成纤维细胞。在所有细胞类型中,SLC30A10 N43A的锰外排活性与野生型相当,而SLC30A10 E25A则缺乏活性。重要的是,与SLC30A10不同,锌运输需要SLC30A1 / ZnT1的HXXXD基序的组氨酸残基。这些结果表明,SLC30A10跨膜结构域内离子配位的机理与先前研究的CDF实质上不同,表明位点A残基以外的因素可能赋予CDF金属特异性,并增进了对锰毒性的病理生物学的认识,这归因于SLC30A10的突变。 SLC30A10。
更新日期:2018-07-04
中文翻译:

锰转运蛋白SLC30A10跨膜结构域的推定金属结合位点与相关的锌转运蛋白不同
SLC30蛋白属于金属转运蛋白的阳离子扩散促进子(CDF)超家族。SLC30A10介导锰流出,而其他SLC30成员则输送锌。CDF的金属特异性可以由形成跨膜金属结合位点的氨基酸赋予(部位A)。含锌的CDF的位点A(例如SLC30A1 / ZnT1)具有HXXXD图案,但是锰转运蛋白(例如SLC30A10)带有NXXXD主题。有人提出在残基43处进行此关键的组氨酸至天冬酰胺关键取代是SLC30A10锰转运特异性的基础。然而,我们最近发现,天冬酰胺43对于HeLa细胞中的锰流出是不可或缺的。相反,需要谷氨酸25,天冬氨酸40,天冬酰胺127和天冬氨酸248。相反,另一小组报告说,鸡细胞系中需要天冬酰胺43。这项研究的目的是解决关于关键天冬酰胺43残留量需求的分歧结果。为此,我们比较了稳定过量表达SLC30A10野生型(WT),SLC30A10 N43A或SLC30A10 E25A的四种细胞类型的锰外流活性:来自Slc30a10 - /-小鼠的与生理相关的肝HepG2和神经元AF5细胞,HEK细胞以及胚胎成纤维细胞。在所有细胞类型中,SLC30A10 N43A的锰外排活性与野生型相当,而SLC30A10 E25A则缺乏活性。重要的是,与SLC30A10不同,锌运输需要SLC30A1 / ZnT1的HXXXD基序的组氨酸残基。这些结果表明,SLC30A10跨膜结构域内离子配位的机理与先前研究的CDF实质上不同,表明位点A残基以外的因素可能赋予CDF金属特异性,并增进了对锰毒性的病理生物学的认识,这归因于SLC30A10的突变。 SLC30A10。









































 京公网安备 11010802027423号
京公网安备 11010802027423号