当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Indenopyridine Derivatives via MgI2‐Promoted [2+4] Cycloaddition Reaction of In‐situ Generated 2‐Styrylmalonate from Donor‐Acceptor Cyclopropanes and Chalconimines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-07-25 , DOI: 10.1002/adsc.201800598 Kamal Verma 1 , Prabal Banerjee 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-07-25 , DOI: 10.1002/adsc.201800598 Kamal Verma 1 , Prabal Banerjee 1
Affiliation
|
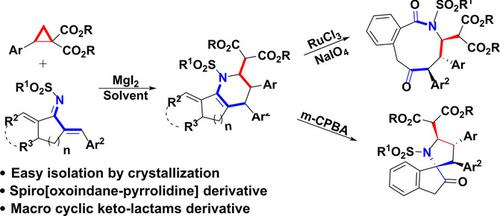
An unexpected MgI2‐promoted [2+4] cycloaddition reaction of in‐situ generated 2‐styrylmalonate from donor‐acceptor cyclopropanes with chalconimines to synthesize highly substituted indenopyridine derivatives under the mild reaction conditions have been developed. Additionally, these derivatives were utilized for the synthesis of highly substituted 9‐membered lactam by oxidative C=C bond cleavage and spiro[oxoindane‐pyrrolidine] derivative via Meinwald type rearrangement.
中文翻译:

MgI2促进的[2 + 4]环加成反应从原位生成的2-苯乙烯基丙二酸酯的供体-受体环丙烷和邻苯二甲酰亚胺合成茚并吡啶衍生物。
已经开发出了出乎意料的MgI 2促进的[2 + 4]环加成反应,该反应是在温和的反应条件下,从供体-受体环丙烷中与硫属元素胺生成2-苯乙烯基丙二酸酯,合成高取代度茚并吡啶衍生物。此外,这些衍生物还用于通过氧化C = C键裂解和Meinwald型重排生成螺[氧代茚满-吡咯烷]衍生物来合成高度取代的9元内酰胺。
更新日期:2018-07-25
中文翻译:

MgI2促进的[2 + 4]环加成反应从原位生成的2-苯乙烯基丙二酸酯的供体-受体环丙烷和邻苯二甲酰亚胺合成茚并吡啶衍生物。
已经开发出了出乎意料的MgI 2促进的[2 + 4]环加成反应,该反应是在温和的反应条件下,从供体-受体环丙烷中与硫属元素胺生成2-苯乙烯基丙二酸酯,合成高取代度茚并吡啶衍生物。此外,这些衍生物还用于通过氧化C = C键裂解和Meinwald型重排生成螺[氧代茚满-吡咯烷]衍生物来合成高度取代的9元内酰胺。









































 京公网安备 11010802027423号
京公网安备 11010802027423号