Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reduction in the Repulsive Forces between Two Charged Surfaces in Aqueous Solutions Containing Salts by a Liquid Flow
Langmuir ( IF 3.7 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1021/acs.langmuir.8b01336 Hayato Kawakami 1 , Cathy E. McNamee 1
Langmuir ( IF 3.7 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1021/acs.langmuir.8b01336 Hayato Kawakami 1 , Cathy E. McNamee 1
Affiliation
|
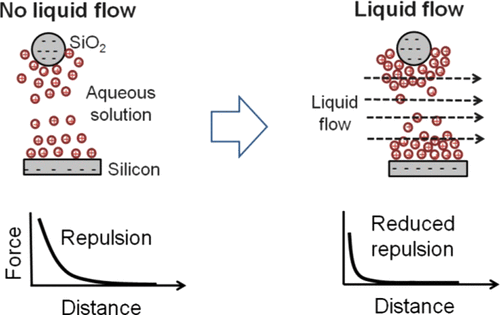
In spite of the fact that a flow is often present in the liquid in which charged particles are dispersed, the effect of a flow on the forces controlling the dispersion is not clear. Here, we used a combined atomic force microscope-peristaltic pump system to determine the effect of a flow in aqueous solutions between a negatively charged silica particle and a negatively charged silicon wafer on the forces in the system. The effect of a flow on the forces in water or aqueous solutions of NaCl or MgCl2·6H2O was studied for salt concentrations lower than the concentrations needed to invert the charge of the silica and silicon surfaces. This was done to prevent the formation of a reversed flow in the system due to a charge inversion of the silica surface. A flow was seen to decrease the intersurface repulsive forces, if the water contained salt (NaCl or MgCl2·6H2O). An increased bulk salt concentration was also seen to decrease the repulsive forces further in the presence of a liquid flow. The surface potentials and effective ionic concentrations of the systems were determined by comparing the experimental curves with the theoretically calculated ones. The surface potentials and effective ionic concentrations were seen to decrease and increase, respectively, as the flow rate and bulk salt concentrations were increased. This change was explained by the shrinking of the diffuse layers by the liquid flow, due to part of the diffuse layer being washed away by the flowing liquid.
中文翻译:

液流降低含盐水溶液中两个带电表面之间的排斥力
尽管事实上在分散有带电粒子的液体中经常存在流动,但不清楚流动对控制分散力的作用。在这里,我们使用了组合的原子力显微镜-蠕动泵系统来确定带负电的二氧化硅颗粒和带负电的硅片之间的水溶液流动对系统中的力的影响。流动对NaCl或MgCl 2 ·6H 2的水溶液中的力的影响研究了O的盐浓度低于使二氧化硅和硅表面的电荷反转所需的盐浓度。这样做是为了防止由于二氧化硅表面的电荷反转而在系统中形成逆流。如果水中含有盐分(NaCl或MgCl 2 ·6H 2O)。在存在液体流的情况下,还发现增加的总盐浓度进一步降低了排斥力。通过将实验曲线与理论计算的曲线进行比较,可以确定系统的表面电势和有效离子浓度。随着流速和盐浓度的增加,表面电势和有效离子浓度分别降低和增加。这种变化是由于液体流动使扩散层收缩而造成的,这是由于扩散层的一部分被流动的液体冲走了。
更新日期:2018-07-03
中文翻译:

液流降低含盐水溶液中两个带电表面之间的排斥力
尽管事实上在分散有带电粒子的液体中经常存在流动,但不清楚流动对控制分散力的作用。在这里,我们使用了组合的原子力显微镜-蠕动泵系统来确定带负电的二氧化硅颗粒和带负电的硅片之间的水溶液流动对系统中的力的影响。流动对NaCl或MgCl 2 ·6H 2的水溶液中的力的影响研究了O的盐浓度低于使二氧化硅和硅表面的电荷反转所需的盐浓度。这样做是为了防止由于二氧化硅表面的电荷反转而在系统中形成逆流。如果水中含有盐分(NaCl或MgCl 2 ·6H 2O)。在存在液体流的情况下,还发现增加的总盐浓度进一步降低了排斥力。通过将实验曲线与理论计算的曲线进行比较,可以确定系统的表面电势和有效离子浓度。随着流速和盐浓度的增加,表面电势和有效离子浓度分别降低和增加。这种变化是由于液体流动使扩散层收缩而造成的,这是由于扩散层的一部分被流动的液体冲走了。











































 京公网安备 11010802027423号
京公网安备 11010802027423号