当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of electrolyte on the nanostructure of the solid electrolyte interphase (SEI) and performance of lithium metal anodes†
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1039/c8ee00364e Sunhyung Jurng 1, 2, 3, 4 , Zachary L. Brown 1, 2, 3, 4 , Jiyeon Kim 1, 2, 3, 4 , Brett L. Lucht 1, 2, 3, 4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1039/c8ee00364e Sunhyung Jurng 1, 2, 3, 4 , Zachary L. Brown 1, 2, 3, 4 , Jiyeon Kim 1, 2, 3, 4 , Brett L. Lucht 1, 2, 3, 4
Affiliation
|
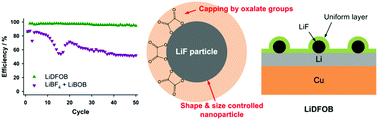
Developing electrolytes that enable commercially viable lithium metal anodes for rechargeable lithium batteries remains challenging, despite recent exhaustive efforts. Electrolytes of similar composition, yet different structure, have been investigated to understand key mechanisms for improving the cycling performance of lithium metal anodes. Specifically, the electrolytes investigated include LiPF6, LiBF4, lithium bis(oxalato)borate (LiBOB), and lithium difluoro(oxalato)borate (LiDFOB) dissolved in a mixture of ethylene carbonate (EC) and ethyl methyl carbonate (EMC). There is a remarkable difference in the cycling performance of 1.2 M LiDFOB in EC : EMC (3 : 7) compared to 0.6 M LiBF4 + 0.6 M LiBOB in EC : EMC (3 : 7), despite the effectively equivalent chemical composition. The LiDFOB electrolyte has significantly better cycling performance. Furthermore, the chemical compositions of the SEI generated on the lithium metal electrode from the two electrolytes are very similar, especially after the 1st plating, suggesting that the chemical composition of the SEI may not be the primary source for the difference in cycling performance. Ex situ transmission electron microscopy (TEM) reveals that the difference in cycling performance can be traced to the presence of nanostructured LiF particles in the SEI from the LiDFOB electrolyte. It is proposed that the capping ability of the oxalate moiety from LiDFOB, in combination with simultaneous generation of LiF, leads to generation of uniform and evenly distributed nanostructured LiF particles. The presence of nanostructured LiF in the SEI results in uniform diffusion field gradients on the lithium electrode which leads to improved cycling performance. The proposed mechanism not only provides insight for improving lithium metal anodes for batteries, but also expands upon the understanding of the role of LiF in the SEI on graphite electrodes in commercial lithium ion batteries. A superior understanding of the structure and function of the SEI will facilitate the development of next-generation energy storage systems.
中文翻译:

电解质对固态电解质相(SEI)纳米结构和锂金属阳极性能的影响†
尽管最近进行了详尽的努力,但开发能够使商业上可行的可再充电锂电池用锂金属阳极的电解质仍然具有挑战性。已经研究了组成相似但结构不同的电解质,以了解改善锂金属阳极循环性能的关键机理。具体地,所研究的电解质包括溶解在碳酸亚乙酯(EC)和碳酸乙酯(EMC)的混合物中的LiPF 6,LiBF 4,双(草酸)硼酸锂(LiBOB)和二氟(草酸)硼酸锂(LiDFOB)。与0.6 M LiBF 4相比,EC:EMC(3:7)中1.2 M LiDFOB的循环性能有显着差异尽管化学成分相当,但EC中的+ 0.6 M LiBOB:EMC(3:7)。LiDFOB电解质具有明显更好的循环性能。此外,由两种电解质在锂金属电极上生成的SEI的化学组成非常相似,尤其是在第一次电镀后,这表明SEI的化学组成可能不是循环性能差异的主要来源。异地透射电子显微镜(TEM)显示,循环性能的差异可归因于LiDFOB电解质在SEI中存在纳米结构LiF颗粒。提出了来自LiDFOB的草酸盐部分的封端能力,与同时产生LiF相结合,导致产生均匀且均匀分布的纳米结构的LiF颗粒。SEI中纳米结构LiF的存在会导致锂电极上均匀的扩散场梯度,从而改善循环性能。提出的机制不仅为改善电池的锂金属阳极提供了见识,而且扩展了对LiF在商业化锂离子电池中石墨电极上SEI中的作用的理解。
更新日期:2018-07-03
中文翻译:

电解质对固态电解质相(SEI)纳米结构和锂金属阳极性能的影响†
尽管最近进行了详尽的努力,但开发能够使商业上可行的可再充电锂电池用锂金属阳极的电解质仍然具有挑战性。已经研究了组成相似但结构不同的电解质,以了解改善锂金属阳极循环性能的关键机理。具体地,所研究的电解质包括溶解在碳酸亚乙酯(EC)和碳酸乙酯(EMC)的混合物中的LiPF 6,LiBF 4,双(草酸)硼酸锂(LiBOB)和二氟(草酸)硼酸锂(LiDFOB)。与0.6 M LiBF 4相比,EC:EMC(3:7)中1.2 M LiDFOB的循环性能有显着差异尽管化学成分相当,但EC中的+ 0.6 M LiBOB:EMC(3:7)。LiDFOB电解质具有明显更好的循环性能。此外,由两种电解质在锂金属电极上生成的SEI的化学组成非常相似,尤其是在第一次电镀后,这表明SEI的化学组成可能不是循环性能差异的主要来源。异地透射电子显微镜(TEM)显示,循环性能的差异可归因于LiDFOB电解质在SEI中存在纳米结构LiF颗粒。提出了来自LiDFOB的草酸盐部分的封端能力,与同时产生LiF相结合,导致产生均匀且均匀分布的纳米结构的LiF颗粒。SEI中纳米结构LiF的存在会导致锂电极上均匀的扩散场梯度,从而改善循环性能。提出的机制不仅为改善电池的锂金属阳极提供了见识,而且扩展了对LiF在商业化锂离子电池中石墨电极上SEI中的作用的理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号