当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoelectron spectroscopic and computational studies of [EDTA·M(iii)]− complexes (M = H3, Al, Sc, V–Co)†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1039/c8cp01548a Qinqin Yuan 1, 2, 3, 4, 5 , Xiang-Tao Kong 1, 2, 3, 4, 5 , Gao-Lei Hou 6, 7, 8, 9 , Ling Jiang 1, 2, 3, 4, 5 , Xue-Bin Wang 6, 7, 8, 9
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1039/c8cp01548a Qinqin Yuan 1, 2, 3, 4, 5 , Xiang-Tao Kong 1, 2, 3, 4, 5 , Gao-Lei Hou 6, 7, 8, 9 , Ling Jiang 1, 2, 3, 4, 5 , Xue-Bin Wang 6, 7, 8, 9
Affiliation
|
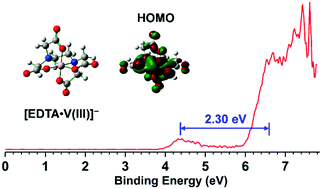
Metal–EDTA complexes commonly exist as biological redox reagents. We have generated a series of such complexes, [EDTA·M(III)]− (M = Al, Sc, V–Co), via electrospray ionization and characterized them by cryogenic mass-selected negative ion photoelectron spectroscopy (NIPES) and quantum chemical computations. Experiments clearly revealed one more spectral band at low electron binding energy for transition metal complexes with d electrons (M = V–Co) compared to those without d electrons (M = Al and Sc). Quantum chemical calculations suggested that all of the metal complexes possess hexacoordinated metal–ligand binding motifs, from which the calculated adiabatic/vertical detachment energy (ADE/VDE) and band gaps are in good agreement with experimental values. Direct spectrum and electronic structure analyses indicted that [EDTA·V(III)]− can be easily oxidized to [EDTA·V(IV)] with the smallest ADE/VDE of 3.95/4.40 eV among these metal complexes, but further oxidation is hindered by the existence of a 2.30 eV band gap, a fact that accords with the special redox behavior of vanadium-containing species in biological cells. Spin density and molecular orbital analyses reveal that [EDTA·V(III)]− was overwhelmingly detached from the vanadium atom, in stark contrast to [EDTA·Sc(III)/Al(III)]−, where the detachment occurred from the EDTA ligand. For all other metal complex anions, from M = Cr to Co, the detachment process is derived from contributions from both the metal and ligand. The intrinsic electronic and geometric structures of these complexes, obtained in this work, provide a molecular foundation to better understand their redox chemistries and specific metal bindings in condensed phases and biological cells.
中文翻译:

[EDTA·M(iii)] -配合物(M = H 3,Al,Sc,V–Co)的光电子能谱和计算研究†
金属-EDTA络合物通常作为生物氧化还原剂存在。我们已经产生一系列这样的配合物,[EDTA·M(的III)] -(M =铝,钪,V-CO),经由电喷雾电离,并通过低温质量选择负离子光电子能谱(NIPES)和量子化学计算对其进行表征。实验清楚地表明,与没有d电子的过渡金属配合物(M = Al和Sc)相比,具有d电子的过渡金属配合物(M = V–Co)在低电子结合能下又有一个光谱带。量子化学计算表明,所有金属配合物均具有六配位的金属-配体结合基序,由此计算出的绝热/垂直脱离能(ADE / VDE)和带隙与实验值非常吻合。直接光谱和电子结构分析表明[EDTA·V(III)] -可以很容易地氧化为[EDTA·V(IV))]在这些金属配合物中的ADE / VDE最小,为3.95 / 4.40 eV,但由于存在2.30 eV带隙,进一步的氧化受到阻碍,这一事实与生物细胞中含钒物质的特殊氧化还原行为相符。 。自旋密度和分子轨道分析表明,[EDTA·V(III)] -与钒原子绝大多数脱附,与[EDTA·Sc(III)/ Al(III)] -形成鲜明对比。,其中发生了与EDTA配体的分离。对于所有其他金属配位阴离子,从M = Cr到Co,其分离过程均来自金属和配体的贡献。通过这项工作获得的这些配合物的内在电子和几何结构,为更好地了解其在还原相和生物细胞中的氧化还原化学性质和特定金属结合提供了分子基础。
更新日期:2018-07-03
中文翻译:

[EDTA·M(iii)] -配合物(M = H 3,Al,Sc,V–Co)的光电子能谱和计算研究†
金属-EDTA络合物通常作为生物氧化还原剂存在。我们已经产生一系列这样的配合物,[EDTA·M(的III)] -(M =铝,钪,V-CO),经由电喷雾电离,并通过低温质量选择负离子光电子能谱(NIPES)和量子化学计算对其进行表征。实验清楚地表明,与没有d电子的过渡金属配合物(M = Al和Sc)相比,具有d电子的过渡金属配合物(M = V–Co)在低电子结合能下又有一个光谱带。量子化学计算表明,所有金属配合物均具有六配位的金属-配体结合基序,由此计算出的绝热/垂直脱离能(ADE / VDE)和带隙与实验值非常吻合。直接光谱和电子结构分析表明[EDTA·V(III)] -可以很容易地氧化为[EDTA·V(IV))]在这些金属配合物中的ADE / VDE最小,为3.95 / 4.40 eV,但由于存在2.30 eV带隙,进一步的氧化受到阻碍,这一事实与生物细胞中含钒物质的特殊氧化还原行为相符。 。自旋密度和分子轨道分析表明,[EDTA·V(III)] -与钒原子绝大多数脱附,与[EDTA·Sc(III)/ Al(III)] -形成鲜明对比。,其中发生了与EDTA配体的分离。对于所有其他金属配位阴离子,从M = Cr到Co,其分离过程均来自金属和配体的贡献。通过这项工作获得的这些配合物的内在电子和几何结构,为更好地了解其在还原相和生物细胞中的氧化还原化学性质和特定金属结合提供了分子基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号