当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
(3 + 3) Annulation of Nitroallylic Acetates with Stabilized Sulfur Ylides for the Synthesis of 2-Aryl Terephthalates
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1021/acs.joc.8b00917 Lakshminarayana Satham 1 , Irishi N. N. Namboothiri 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1021/acs.joc.8b00917 Lakshminarayana Satham 1 , Irishi N. N. Namboothiri 1
Affiliation
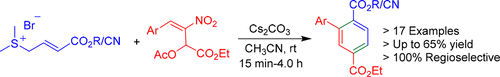
|
A novel (3 + 3) annulation approach has been developed for the synthesis of 2-aryl terephthalates from nitroallylic acetates and stabilized sulfur ylides. The 2-aryl terephthalates, which are also biaryls bearing a terephthalate moiety, are formed through a cascade of reactions such as a γ-selective SN2′ reaction, γ-selective intramolecular Michael addition, and two eliminations in the presence of Cs2CO3 in CH3CN at room temperature. The products are formal precursors of farnesyltransferase inhibitors and are also potential monomers in polymer chemistry.
中文翻译:

(3 + 3)硝基烯丙基乙酸盐与稳定的硫磺盐合成2-对苯二甲酸对苯二甲酸酯的合成
已经开发了一种新颖的(3 + 3)环空方法,用于从硝基烯丙基乙酸酯和稳定的硫酰化物合成对苯二甲酸2-芳基酯。的2-芳基对苯二甲酸酯,其也是联芳基带有对苯二甲酸乙二醇酯部分,通过反应器的级联形成诸如γ-选择性小号Ñ 2'反应,γ-选择性分子内迈克尔加成和两个抵销在Cs的存在2室温下CH 3 CN中的CO 3。该产品是法尼基转移酶抑制剂的正式前体,也是聚合物化学中的潜在单体。
更新日期:2018-07-03
中文翻译:

(3 + 3)硝基烯丙基乙酸盐与稳定的硫磺盐合成2-对苯二甲酸对苯二甲酸酯的合成
已经开发了一种新颖的(3 + 3)环空方法,用于从硝基烯丙基乙酸酯和稳定的硫酰化物合成对苯二甲酸2-芳基酯。的2-芳基对苯二甲酸酯,其也是联芳基带有对苯二甲酸乙二醇酯部分,通过反应器的级联形成诸如γ-选择性小号Ñ 2'反应,γ-选择性分子内迈克尔加成和两个抵销在Cs的存在2室温下CH 3 CN中的CO 3。该产品是法尼基转移酶抑制剂的正式前体,也是聚合物化学中的潜在单体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号