当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tuning the K+ Concentration in the Tunnels of α-MnO2 To Increase the Content of Oxygen Vacancy for Ozone Elimination
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-07-17 , DOI: 10.1021/acs.est.8b01594 Guoxiang Zhu 1 , Jinguo Zhu 2 , Wenlu Li 1 , Wenqing Yao 1 , Ruilong Zong 1 , Yongfa Zhu 1 , Qianfan Zhang 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-07-17 , DOI: 10.1021/acs.est.8b01594 Guoxiang Zhu 1 , Jinguo Zhu 2 , Wenlu Li 1 , Wenqing Yao 1 , Ruilong Zong 1 , Yongfa Zhu 1 , Qianfan Zhang 2
Affiliation
|
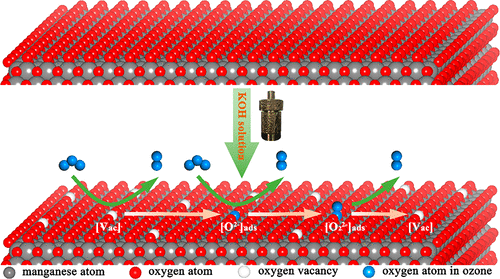
α-MnO2 is a promising material for ozone catalytic decomposition and the oxygen vacancy is often regarded as the active site for ozone adsorption and decomposition. Here, α-MnO2 nanowire with tunable K+ concentration was prepared through a hydrothermal process in KOH solution. High concentration K+ in the tunnel can expand crystal cell and break the charge balance, leading to a lower average oxidation state (AOS) of Mn, which means abundant oxygen vacancy. DFT calculation has also proven that the samples with higher K+ concentration exhibit lower formation energy for oxygen vacancy. Due to the enormous active oxygen vacancies existing in the α-MnO2 nanowire, the lifetime of the catalyst (corresponding to 100% ozone removal rate, 25 °C) is increased from 3 to 15 h. The FT-IR results confirmed that the accumulation of intermediate oxygen species on the catalyst surface is the main reason why it is deactivated after long time reaction. In this work, the performance of the catalyst has been improved because the abundant active oxygen vacancies are fabricated by the electrostatic interaction between oxygen atoms inside the tunnels and the introduced K+, which offers us a new perspective to design a high efficiency catalyst and may promote manganese oxide for practical ozone elimination.
中文翻译:

调谐ķ + α-MnO的的隧道浓度2为了增加氧空位的臭氧消除的内容
α-MnO的2为臭氧催化分解有希望的材料和氧空位通常被认为是活性位点的臭氧吸附和分解。这里,α-MnO的2纳米线具有可调谐ķ +浓度通过在KOH溶液中水热法来制备。隧道中的高浓度K +可以扩展晶体晶胞并破坏电荷平衡,从而导致Mn的平均氧化态(AOS)降低,这意味着大量的氧空位。DFT计算还证明,具有较高K +浓度的样品显示出较低的空位形成能。由于存在于α-MnO的巨大活性氧空位2纳米线,催化剂的寿命(对应于25%的100%臭氧去除率)从3小时增加到15小时。FT-IR结果证实,中间氧物种在催化剂表面上的积累是长时间反应后其失活的主要原因。在这项工作中,由于隧道内的氧原子与引入的K +之间的静电相互作用产生了大量的活性氧空位,从而提高了催化剂的性能,这为我们设计高效催化剂提供了新的视角,并且可能促进锰氧化物的实际消除臭氧。
更新日期:2018-07-18
中文翻译:

调谐ķ + α-MnO的的隧道浓度2为了增加氧空位的臭氧消除的内容
α-MnO的2为臭氧催化分解有希望的材料和氧空位通常被认为是活性位点的臭氧吸附和分解。这里,α-MnO的2纳米线具有可调谐ķ +浓度通过在KOH溶液中水热法来制备。隧道中的高浓度K +可以扩展晶体晶胞并破坏电荷平衡,从而导致Mn的平均氧化态(AOS)降低,这意味着大量的氧空位。DFT计算还证明,具有较高K +浓度的样品显示出较低的空位形成能。由于存在于α-MnO的巨大活性氧空位2纳米线,催化剂的寿命(对应于25%的100%臭氧去除率)从3小时增加到15小时。FT-IR结果证实,中间氧物种在催化剂表面上的积累是长时间反应后其失活的主要原因。在这项工作中,由于隧道内的氧原子与引入的K +之间的静电相互作用产生了大量的活性氧空位,从而提高了催化剂的性能,这为我们设计高效催化剂提供了新的视角,并且可能促进锰氧化物的实际消除臭氧。











































 京公网安备 11010802027423号
京公网安备 11010802027423号