当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereospecific Radical-Mediated B12-Dependent Methyl Transfer by the Fosfomycin Biosynthesis Enzyme Fom3
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1021/acs.biochem.8b00616 Martin I McLaughlin , Wilfred A van der Donk
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1021/acs.biochem.8b00616 Martin I McLaughlin , Wilfred A van der Donk
|
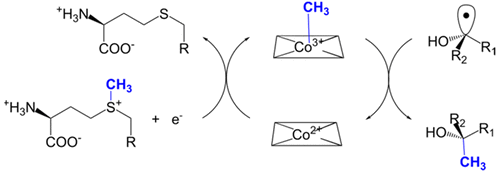
Fom3, the antepenultimate enzyme in the fosfomycin biosynthetic pathway in Streptomyces spp., is a class B cobalamin-dependent radical SAM methyltransferase that catalyzes methylation of (5′-cytidylyl)-2-hydroxyethylphosphonate (2-HEP-CMP) to form (5′-cytidylyl)-2-hydroxypropylphosphonate (2-HPP-CMP). Previously, the reaction of Fom3 with 2-HEP-CMP produced 2-HPP-CMP with mixed stereochemistry at C2. Mechanistic characterization has been challenging because of insoluble expression and poor cobalamin (B12) incorporation in Escherichia coli. Recently, soluble E. coli expression and incorporation of cobalamin into Fom3 were achieved by overexpression of the BtuCEDFB cobalamin uptake system. Herein, we use this new method to obtain Fom3 from Streptomyces wedmorensis. We show that the initiator 5′-deoxyadenosyl radical stereospecifically abstracts the pro-R hydrogen atom from the C2 position of 2-HEP-CMP and use the downstream enzymes FomD and Fom4 to demonstrate that our preparation of Fom3 produces only (2S)-2-HPP-CMP. Additionally, we show that the added methyl group originates from SAM under multiple-turnover conditions, but the first turnover uses a methyl donor already present on the enzyme; furthermore, cobalamin isolated from Fom3 reaction mixtures contains methyl groups derived from SAM. These results are consistent with a model in which Fom3 catalyzes methyl transfer from SAM to cobalamin and the resulting methylcobalamin (MeCbl) is the ultimate methyl source for the reaction.
中文翻译:

磷霉素生物合成酶 Fom3 立体特异性自由基介导的 B12 依赖性甲基转移
Fom3 是链霉菌中磷霉素生物合成途径中的倒数第二个酶,是一种 B 类钴胺素依赖性自由基 SAM 甲基转移酶,可催化 (5'-胞苷基)-2-羟乙基膦酸酯 (2-HEP-CMP) 甲基化形成 (5 '-胞苷基)-2-羟丙基膦酸酯 (2-HPP-CMP)。此前,Fom3 与 2-HEP-CMP 反应产生了 C2 处具有混合立体化学的 2-HPP-CMP。由于大肠杆菌中的不溶性表达和钴胺素 (B 12 ) 掺入不良,机制表征一直具有挑战性。最近,通过过度表达 BtuCEDFB 钴胺素摄取系统,实现了可溶性大肠杆菌表达并将钴胺素掺入 Fom3。在此,我们使用这种新方法从Streptomyces wedmorensis中获得Fom3。我们展示了引发剂 5'-脱氧腺苷基自由基从 2-HEP-CMP 的 C2 位置立体定向地提取了前R氢原子,并使用下游酶 FomD 和 Fom4 来证明我们制备的 Fom3 只产生 (2 S )- 2-HPP-CMP。此外,我们还表明,在多次周转条件下,添加的甲基源自 SAM,但第一次周转使用了酶上已经存在的甲基供体;此外,从 Fom3 反应混合物中分离的钴胺素含有源自 SAM 的甲基。这些结果与 Fom3 催化甲基从 SAM 转移到钴胺素的模型一致,所得的甲基钴胺素 (MeCbl) 是该反应的最终甲基来源。
更新日期:2018-07-03
中文翻译:

磷霉素生物合成酶 Fom3 立体特异性自由基介导的 B12 依赖性甲基转移
Fom3 是链霉菌中磷霉素生物合成途径中的倒数第二个酶,是一种 B 类钴胺素依赖性自由基 SAM 甲基转移酶,可催化 (5'-胞苷基)-2-羟乙基膦酸酯 (2-HEP-CMP) 甲基化形成 (5 '-胞苷基)-2-羟丙基膦酸酯 (2-HPP-CMP)。此前,Fom3 与 2-HEP-CMP 反应产生了 C2 处具有混合立体化学的 2-HPP-CMP。由于大肠杆菌中的不溶性表达和钴胺素 (B 12 ) 掺入不良,机制表征一直具有挑战性。最近,通过过度表达 BtuCEDFB 钴胺素摄取系统,实现了可溶性大肠杆菌表达并将钴胺素掺入 Fom3。在此,我们使用这种新方法从Streptomyces wedmorensis中获得Fom3。我们展示了引发剂 5'-脱氧腺苷基自由基从 2-HEP-CMP 的 C2 位置立体定向地提取了前R氢原子,并使用下游酶 FomD 和 Fom4 来证明我们制备的 Fom3 只产生 (2 S )- 2-HPP-CMP。此外,我们还表明,在多次周转条件下,添加的甲基源自 SAM,但第一次周转使用了酶上已经存在的甲基供体;此外,从 Fom3 反应混合物中分离的钴胺素含有源自 SAM 的甲基。这些结果与 Fom3 催化甲基从 SAM 转移到钴胺素的模型一致,所得的甲基钴胺素 (MeCbl) 是该反应的最终甲基来源。











































 京公网安备 11010802027423号
京公网安备 11010802027423号