当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Designed Self-Assembly of Peptides with G-Quadruplex/Hemin DNAzyme into Nanofibrils Possessing Enzyme-Mimicking Active Sites and Catalytic Functions
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1021/acscatal.8b00896 Zhen-Gang Wang 1 , Hui Wang 1 , Qing Liu 1 , Fangyuan Duan 1 , Xinghua Shi 1, 2 , Baoquan Ding 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1021/acscatal.8b00896 Zhen-Gang Wang 1 , Hui Wang 1 , Qing Liu 1 , Fangyuan Duan 1 , Xinghua Shi 1, 2 , Baoquan Ding 1, 2
Affiliation
|
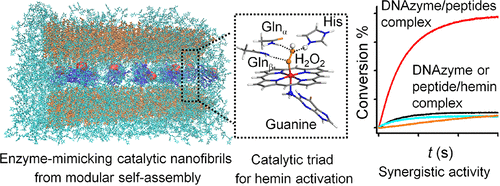
Enzymes fold into three-dimensional structures to arrange their active groups exquisitely for the remarkable catalytic properties. We are inspired to design and assemble Gln-containing peptides with G-quadruplex DNA/hemin complexes to form the catalytic nanofibrils that possess the horseradish peroxidase-mimicking active sites and catalytic functions. Theoretical simulation results revealed that the intermolecular association of Gln peptide may result in local enrichment and proper orientation of carboxamide groups, which provide potential multivalent hydrogen bonds for enhancing H2O2 affinity to hemin and may behave similarly to distal Arg in a natural heme pocket. The self-folded DNA can provide a guanine base as the axial ligand, and a supramolecular scaffold for supporting and orienting hemin. The β-sheet forming capability of the Q peptides is found to significantly affect the catalytic synergy between the G-DNA and the peptide. The role of hydrogen bonds network provided by self-assembled Gln peptides is illustrated by solvent kinetic isotope effects and H2O2-induced degradation of hemin. The assembly of the Q peptide with G-DNA/hemin DNAzyme also stimulates the chiroselective oxidization of L-DOPA vs D-DOPA. The incorporation of His-containing peptide into the hemin system via self-assembly, which was demonstrated by confocal colocalization images and fluorescence resonance electron transfer results, further enhanced the catalytic activity of the G-DNA/Q peptide/hemin complex. It is hypothesized that the assembly of the triple components allows mimicking the configuration and the function of the catalytic His-Arg-His triad in horseradish peroxidase. Compared to DNA/hemin or peptide/hemin, the catalytic efficiency of the most active complex shows 10-fold enhanced activity. This work opens an avenue to mimic the catalytic residues and their spatial distribution and may provide a primitive enzyme model for the evolution of modern enzymes. Our results may also have implications for the mechanisms of some cell dysfunctions, which are triggered by catalytic aggregation of biomolecules.
中文翻译:

设计的肽自组装与G四联体/ Hemin DNAzyme到纳米纤丝,具有模仿酶的活性位点和催化功能。
酶折叠成三维结构以精确地排列其活性基团,从而具有非凡的催化性能。我们受到启发,设计并组装了含有Gln四联体DNA /血红素复合物的Gln肽,以形成具有类似于辣根过氧化物酶的活性位点和催化功能的催化纳米原纤维。理论模拟结果表明,Gln肽的分子间缔合可能导致羧酰胺基团的局部富集和正确取向,从而为增强H 2 O 2提供潜在的多价氢键。对血红素具有亲和力,在天然血红素袋中的行为可能类似于远端Arg。自折叠的DNA可以提供鸟嘌呤碱基作为轴向配体,并提供用于支持和定向血红素的超分子支架。发现Q肽的β-折叠形成能力显着影响G-DNA和肽之间的催化协同作用。自组装的Gln肽提供的氢键网络的作用通过溶剂动力学同位素效应和H 2 O 2来说明。诱导的血红素降解。Q肽与G-DNA / hemin DNAzyme的组装也刺激L-DOPA与D-DOPA的手性选择性氧化。共聚焦共定位图像和荧光共振电子转移结果证明,通过自组装将含His的肽结合到hemin系统中,进一步增强了G-DNA / Q肽/ hemin复合物的催化活性。假设三组分的组装允许模仿辣根过氧化物酶中催化的His-Arg-His三联体的构型和功能。与DNA /血红素或肽/血红素相比,活性最高的复合物的催化效率提高了10倍。这项工作为模仿催化残基及其空间分布开辟了一条途径,并可能为现代酶的进化提供原始的酶模型。我们的结果也可能对某些细胞功能异常的机制有影响,这些功能异常是由生物分子的催化聚集触发的。
更新日期:2018-07-03
中文翻译:

设计的肽自组装与G四联体/ Hemin DNAzyme到纳米纤丝,具有模仿酶的活性位点和催化功能。
酶折叠成三维结构以精确地排列其活性基团,从而具有非凡的催化性能。我们受到启发,设计并组装了含有Gln四联体DNA /血红素复合物的Gln肽,以形成具有类似于辣根过氧化物酶的活性位点和催化功能的催化纳米原纤维。理论模拟结果表明,Gln肽的分子间缔合可能导致羧酰胺基团的局部富集和正确取向,从而为增强H 2 O 2提供潜在的多价氢键。对血红素具有亲和力,在天然血红素袋中的行为可能类似于远端Arg。自折叠的DNA可以提供鸟嘌呤碱基作为轴向配体,并提供用于支持和定向血红素的超分子支架。发现Q肽的β-折叠形成能力显着影响G-DNA和肽之间的催化协同作用。自组装的Gln肽提供的氢键网络的作用通过溶剂动力学同位素效应和H 2 O 2来说明。诱导的血红素降解。Q肽与G-DNA / hemin DNAzyme的组装也刺激L-DOPA与D-DOPA的手性选择性氧化。共聚焦共定位图像和荧光共振电子转移结果证明,通过自组装将含His的肽结合到hemin系统中,进一步增强了G-DNA / Q肽/ hemin复合物的催化活性。假设三组分的组装允许模仿辣根过氧化物酶中催化的His-Arg-His三联体的构型和功能。与DNA /血红素或肽/血红素相比,活性最高的复合物的催化效率提高了10倍。这项工作为模仿催化残基及其空间分布开辟了一条途径,并可能为现代酶的进化提供原始的酶模型。我们的结果也可能对某些细胞功能异常的机制有影响,这些功能异常是由生物分子的催化聚集触发的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号