当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Surface Hydroxyl Species in Copper-Catalyzed Hydrogenation of Ketones
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1021/acscatal.8b01652 Jenoff E. De Vrieze 1 , Joris W. Thybaut 1 , Mark Saeys 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1021/acscatal.8b01652 Jenoff E. De Vrieze 1 , Joris W. Thybaut 1 , Mark Saeys 1
Affiliation
|
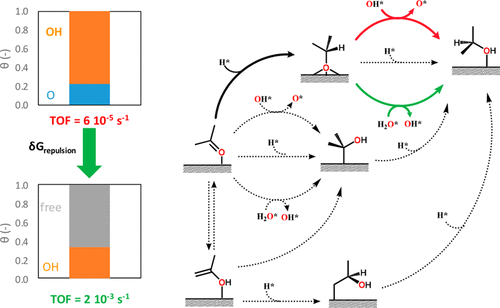
A comprehensive, coverage-dependent mean-field microkinetic model is developed for the hydrogenation of carbonyl compounds on Cu(111). In the model, hydrogenation by surface hydrogen, surface hydroxyl species, and adsorbed water molecules is considered, including a reaction pathway via keto–enol tautomerization. The model parameters were calculated by VdW-DF2 density functional theory and account for inter- and intraspecies repulsion. Accounting for these coverage effects changes the surface from being completely covered with 25% oxygen atoms and 75% hydroxyl groups to a surface with 65% free sites. Including coverage effects also surprisingly increases the calculated turnover frequency from 6 × 10–5 to 2 × 10–3 s–1. In the dominant reaction path, the carbonyl group is hydrogenated to an alkoxy intermediate by surface hydrogen, followed by a proton transfer from either a surface hydroxyl species or an adsorbed water molecule to form the alcohol product. The addition of small amounts of water suffices to open this pathway. The pathway in which acetone is converted to 2-hydroxypropylene via keto–enol tautomerization is kinetically irrelevant under the considered conditions. Regeneration of the hydroxyl groups is the rate-controlling step in the mechanism, suggesting an alternative role for the reducible oxide promoters which are often encountered for Cu-based carbonyl hydrogenation catalysts.
中文翻译:

表面羟基物种在铜催化的酮加氢中的作用
建立了一个全面的,依赖于覆盖率的平均场微动力学模型,用于Cu(111)上羰基化合物的加氢反应。在该模型中,考虑了通过表面氢,表面羟基物质和吸附的水分子进行的氢化,包括通过酮-烯醇互变异构的反应途径。通过VdW-DF2密度泛函理论计算模型参数,并考虑种间和种内排斥。考虑到这些覆盖效应,会将表面从被25%的氧原子和75%的羟基完全覆盖,变为具有65%的自由位点的表面。包括覆盖范围的影响也令人惊讶地将计算出的周转频率从6×10 –5增加到2×10 –3 s –1。在主要的反应路径中,羰基被表面氢氢化成烷氧基中间体,然后从表面羟基物质或吸附的水分子转移质子,形成醇产物。添加少量水就足以打开该路径。在所考虑的条件下,丙酮经酮-烯醇互变异构转化为2-羟基丙烯的途径在动力学上无关紧要。羟基的再生是该机理中的速率控制步骤,表明对于可还原的氧化物促进剂的替代作用,这是基于铜的羰基加氢催化剂经常遇到的。
更新日期:2018-07-03
中文翻译:

表面羟基物种在铜催化的酮加氢中的作用
建立了一个全面的,依赖于覆盖率的平均场微动力学模型,用于Cu(111)上羰基化合物的加氢反应。在该模型中,考虑了通过表面氢,表面羟基物质和吸附的水分子进行的氢化,包括通过酮-烯醇互变异构的反应途径。通过VdW-DF2密度泛函理论计算模型参数,并考虑种间和种内排斥。考虑到这些覆盖效应,会将表面从被25%的氧原子和75%的羟基完全覆盖,变为具有65%的自由位点的表面。包括覆盖范围的影响也令人惊讶地将计算出的周转频率从6×10 –5增加到2×10 –3 s –1。在主要的反应路径中,羰基被表面氢氢化成烷氧基中间体,然后从表面羟基物质或吸附的水分子转移质子,形成醇产物。添加少量水就足以打开该路径。在所考虑的条件下,丙酮经酮-烯醇互变异构转化为2-羟基丙烯的途径在动力学上无关紧要。羟基的再生是该机理中的速率控制步骤,表明对于可还原的氧化物促进剂的替代作用,这是基于铜的羰基加氢催化剂经常遇到的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号