当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of transition-metal-ion dopants on the oxygen evolution reaction on NiOOH(0001)†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1039/c8cp02849d Alexander J. Tkalych 1, 2, 3, 4 , John Mark P. Martirez 2, 3, 4, 5 , Emily A. Carter 2, 3, 4, 6
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-07-03 00:00:00 , DOI: 10.1039/c8cp02849d Alexander J. Tkalych 1, 2, 3, 4 , John Mark P. Martirez 2, 3, 4, 5 , Emily A. Carter 2, 3, 4, 6
Affiliation
|
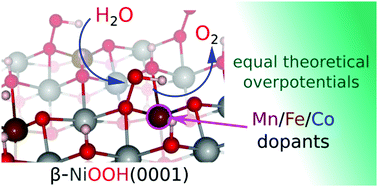
Iron-doped nickel oxyhydroxide has been identified as one of the most active alkaline oxygen evolution reaction (OER) catalysts, exhibiting an overpotential lower than values observed for state-of-the-art precious metal catalysts. Several computational investigations have found widely varying effects of doping on the theoretical overpotential of the OER on NiOx. Comparisons of these results are made difficult by the numerous differences in the structural and computational parameters used in these studies. In this work, within a consistent framework, we calculate the theoretical overpotentials for reactions occurring on the most stable, basal plane of undoped and doped β-NiOOH. We compare the activities of Fe(III), Co(III), and Mn(III) doping using density functional theory with Hubbard-like U corrections on the transition-metal d orbitals. We compare the effect of surface and subsurface doping in order to establish whether the dopants act as new active sites for the reaction or whether they induce more widespread changes in the material. The results of our study find only a small reduction in the overpotential (∼0.1 and ≤0.05 V when doped in the surface and subsurface layers, respectively) for the three dopants, if doped in the dominant basal plane. This is much less than the reductions of 0.3 V experimentally observed for the most active Fe-doped systems. Furthermore, the magnitudes of reductions in overpotentials for the three dopants are similar. This work therefore disqualifies the possibility of enhancing the activity of the dominant exposed basal plane of β-NiOOH through substitutional doping.
中文翻译:

过渡金属离子掺杂剂对NiOOH(0001)上的氧释放反应的影响†
铁掺杂的羟基氧化镍已被确定为最具活性的碱性氧逸出反应(OER)催化剂之一,其超电势低于最先进的贵金属催化剂所观察到的值。几项计算研究发现,掺杂对NiO x上OER的理论超电势的影响各不相同。这些研究中使用的结构和计算参数存在众多差异,因此很难对这些结果进行比较。在这项工作中,我们在一个一致的框架内,计算了在未掺杂和掺杂的β-NiOOH的最稳定,基本平面上发生的反应的理论超电势。我们比较了Fe(III),Co(III)和Mn(III)的活性用密度泛函理论对类哈伯U进行掺杂过渡金属d轨道的修正。我们比较表面和次表面掺杂的影响,以确定掺杂剂是否充当反应的新活性位或它们是否引起材料中更广泛的变化。我们的研究结果发现,如果掺杂在显性基底平面中,则三种掺杂剂的过电势仅会小幅降低(分别在表面层和亚表面层掺杂时分别约为0.1 V和≤0.05 V)。这远远小于最活跃的铁掺杂系统实验观察到的0.3 V的降低。此外,三种掺杂剂的过电势减小的幅度是相似的。因此,这项工作使通过替代掺杂来增强β-NiOOH的主要暴露基面的活性的可能性丧失了。
更新日期:2018-07-03
中文翻译:

过渡金属离子掺杂剂对NiOOH(0001)上的氧释放反应的影响†
铁掺杂的羟基氧化镍已被确定为最具活性的碱性氧逸出反应(OER)催化剂之一,其超电势低于最先进的贵金属催化剂所观察到的值。几项计算研究发现,掺杂对NiO x上OER的理论超电势的影响各不相同。这些研究中使用的结构和计算参数存在众多差异,因此很难对这些结果进行比较。在这项工作中,我们在一个一致的框架内,计算了在未掺杂和掺杂的β-NiOOH的最稳定,基本平面上发生的反应的理论超电势。我们比较了Fe(III),Co(III)和Mn(III)的活性用密度泛函理论对类哈伯U进行掺杂过渡金属d轨道的修正。我们比较表面和次表面掺杂的影响,以确定掺杂剂是否充当反应的新活性位或它们是否引起材料中更广泛的变化。我们的研究结果发现,如果掺杂在显性基底平面中,则三种掺杂剂的过电势仅会小幅降低(分别在表面层和亚表面层掺杂时分别约为0.1 V和≤0.05 V)。这远远小于最活跃的铁掺杂系统实验观察到的0.3 V的降低。此外,三种掺杂剂的过电势减小的幅度是相似的。因此,这项工作使通过替代掺杂来增强β-NiOOH的主要暴露基面的活性的可能性丧失了。











































 京公网安备 11010802027423号
京公网安备 11010802027423号